Onasia
ANTI EMETICS: Ondansetron
Indication
1. Nausea-vomiting associated with gastroenteritis
2. Nausea-vomiting associated with pregnancy
3. Prevention of postoperative nausea and vomiting
4. Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy
5. Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy
6. Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen or daily fractions to the abdomen
Contraindication
Ondansetron is contraindicated for patients known to have hypersensitivity to the drug.
Dosage & Administration
Postoperative nausea and vomiting:
The recommended dose is 16 mg as two 8 mg Onasia tablets 1 hour before induction of anesthesia.
Chemotherapy induced nausea and vomiting:
a. For highly emetogenic cancer chemotherapy:
The recommended adult oral dose is three 8 mg Onasia tablets administered 30 minutes before the start of single day highly emetogenic chemotherapy.
Geriatric use same as the general population.
b. For moderately emetogenic cancer chemotherapy:
The recommended adult oral dose is one 8 mg Onasia tablet given twice a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with a subsequent dose 8 hours after the first dose and Onasia 8 mg tablet should be administered twice a day (every 12 hours) for 1 to 2 days after completion of chemotherapy.
Radiotherapy induced nausea and vomiting:
The recommended oral dose is one 8 mg Onasia tablet given three times a day.
For total body irradiation, one 8 mg Onasia tablet should be administered 1 to 2 hours before each fraction of radiotherapy administered each day. For single high dose fraction radiotherapy to the abdomen, one 8 mg Onasia tablet should be administered 1 to 2 hours before radiotherapy with subsequent doses every 8 hours after the first dose for 1 to 2 days after completion of radiotherapy. For daily fractionated radiotherapy in the abdomen, one 8 mg Onasia tablet should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for each day.
For paediatric patients 4 to 11 years of age, the dosage is one half of Onasia 8 mg tablet given three times a day (every 8 hours). For oral solution, 4 mg (5 ml oral solution) should be administered 3 times a day (Every 8 hours).
Dosage adjustment for patients with impaired renal function:
The dosage recommendation is the same as for the general population.
Dosage adjustment for patients with impaired hepatic function:
In patients with severe hepatic impairment, a total daily dose of 8 mg should not be exceeded.
For Injection
Prevention of Chemotherapy-Induced Nausea and Vomiting: the recommended IV/IM dosage of Onasia is a single 32-mg dose or three 0.15 mg/kg doses. A single 32 mg dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. Subsequent doses (0.15 mg/kg) are administered 4 and 8 hours after the first dose of Onasia.
Pediatric Use
The dosage in pediatric patients 4 to 18 years of age should be three 0.15 mg/kg doses.
Prevention of Postoperative Nausea and Vomiting: The recommended IV/IM dosage of Onasia for adults is 4 mg undiluted administered intravenously in not less than 30 seconds, preferably over 2 to 5 minutes, immediately before induction of anesthesia, or postoperatively if the patient experiences nausea and/or vomiting occurring shortly after surgery. In patients who do not achieve adequate control of postoperative nausea and vomiting following a single, prophylactic, preinduction, IV/IM dose of 4 mg ondansetron postoperatively does not provide additional control of nausea and vomiting.
Side Effect
Generally Ondansetron is well tolerated. However few side effects including headache, diarrhoea, fatigue, dizziness and constipation may be seen after Ondansetron is administered.
Precaution
Ondansetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of ondansetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distension.
Drug Interaction
Pregnancy: Pregnancy category B.
Nursing mother: It is not known whether Ondansetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Ondansetron is administered to a nursing woman.
Presentation
The following drugs should be used with caution when concomitantly used with Ondansetron: Phenytoin, Carbamazepine, Rifampicin & Tramadol.
Contraindications
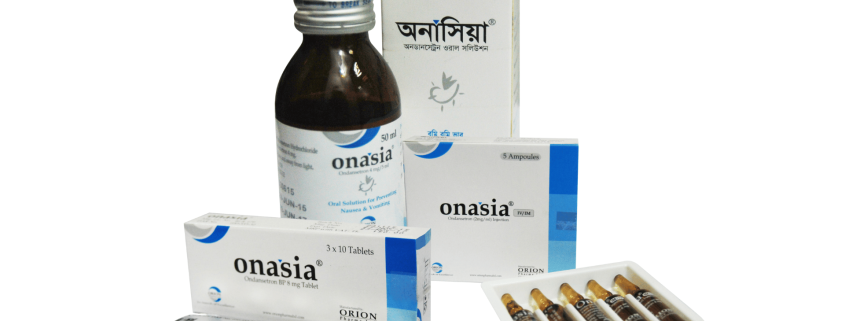
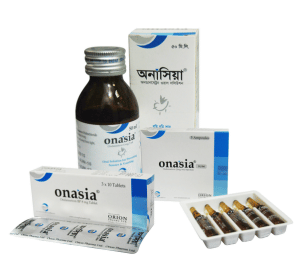
Onasia 8 mg Tablet: Each box contains 3×10 tablets in blister pack.
Onasia 50 ml oral solution: Each bottle contains 50 ml oral solution.
Onasia 4 ml IV/IM injection: Each box contains 5 ampoules of 4 ml.
ANTI EMETICS: Ondansetron