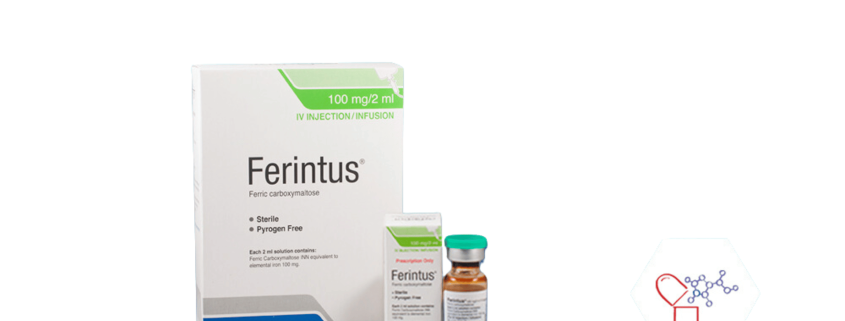
Ferintus
Generic Name: Ferric Carboxymaltose
Dosage Form: IV Injection/Infusion
TG Name: Vitamins & Minerals Supplement
What Ferintus® is and what it is used for?
Ferric carboxymaltose is a iron replacement product indicated for the treatment of iron deficiency anemia in adult patients who have intolerance to oral iron or have had unsatisfactory response to oral iron or those who have non-dialysis dependent chronic kidney disease.
How to take Ferintus®
The cumulative dose for repletion of iron using Ferric Carboxymaltose is determined based on the patient’s body weight and haemoglobin (Hb) level and must not be exceeded. Note: A cumulative iron dose of 500 mg should not be exceeded for patients with a body weight 14 g/dL, an initial dose of 500 mg iron should be given and iron parameters should be checked prior to repeat dosing. Post repletion, regular assessments should be completed to ensure that iron levels are corrected and maintained. A single dose Ferric Carboxymaltose should not exceed 1,000 mg of iron per day. Do not administer 1,000 mg of iron more than once a week.
Possible side effects
The side effects of Ferric Carboxymaltose are infrequent, usually mild and generally do not cause patients to stop treatment. The most common side effects are nausea, injection site reactions (including pain or bruising at the injection site), and asymptomatic reductions in blood phosphorus, flushing, headache, hypertension, dizziness, and increased alanine aminotransferase. Potentially long lasting brown staining of skin near injection site may occur. Uncommon side effects are hypersensitivity, paraesthesia, dyspepsia, tachycardia, hypotension, flushing, dyspnoea, vomiting, dyspepsia, abdominal pain, constipation, diarrhea, pruritus, urticaria, erythema, rash, myalgia, back pain, arthralgia, muscle spasm, pyrexia, fatigue, chest pain, oedema peripheral, chills, aspartate aminotransferase increased, gamma-glutamyltransferase increased, blood lactate dehydrogenase increased and blood alkaline phosphatase increased. Very side effects are anaphylactoid reactions, loss of consciousness, anxiety, phlebitis, syncope, presyncope, bronchospasm, flatulence, angioedema, pallor face oedema, Rigors, malaise and influenza like illness.
Pregnancy and Lactation
A careful risk/benefit evolution is required before use during pregnancy and Ferric Carboxymaltose should not be used during pregnancy unless clearly necessary. Lactating mothers Ferric Carboxymaltose is excreted in human milk which unlikely to affect the baby.
Warnings & Precaution
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Ferric Carboxymaltose. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse.
How to store Ferintus®
Do not store above 30°C prior to opening. Do not freeze.