Vonion

ANTIULCERANTS: Vonoprazan
Indication
Vonion is indicated for
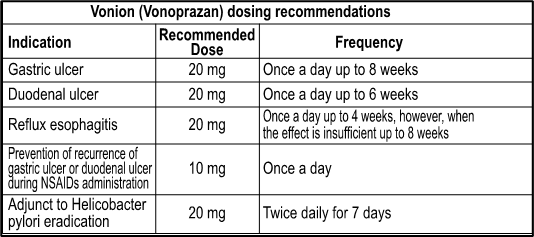
1. Gastric ulcer 2. Duodenal ulcer 3. Reflux esophagitis 4. Prevention of recurrence of gastric ulcer or duodenal ulcer during NSAIDs administration 5. Adjunct to Helicobacter pylori eradication
Contraindication
Vonoprazan hypersensitivity to the active ingredients or to any of the excipients.
Side Effect
Diarrhea, abdominal pain, nausea, upper respiratory tract infection, vomiting & flatulence.
Dosage & Administration
Use Pregnancy Lactation
Pregnant women : No clinical studies have been conducted to date to evaluate vonoprazan in subjects who are pregnant. In a rat toxicology study, embryofetal toxicity was observed following exposure of more than approximately 28 times of the exposure (AUC) at the maximum clinical dose (40 mg/day) of Vonoprazan. Vonoprazan should not be administered to women who are or may be pregnant, unless the expected therapeutic benefit is thought to outweigh any possible risk.
Lactating Mother: No clinical studies have been conducted to date to evaluate vonoprazan in subjects who are lactating. It is unknown whether vonoprazan is excreted in human milk. In animal studies it has been shown that vonoprazan was excreted in milk. During treatment with vonoprazan, nursing should be avoided if the administration of this drug is necessary for the mother.
Storage
Store below 30 ºC, keep in a dry place and protected from light. Keep all medicines out of reach of children.