Virux® IV Injection
Acyclovir USP
(Systemic Antiviral)
Indication:
The following are the indications of Virux® 500 IV Injection:
- Herpes simplex encephalitis
- Acute clinical manifestations of mucocutaneous
- Herpes simplex virus infections in immunocompromised patients
- Severe primary or non-primary genital herpes in immune-competent patients
- Varicella zoster virus infection in immunocompromised patients
- Herpes zoster (shingles) in immune-competent patients who show very severe acute local or systemic manifestations of the disease
Dosage & Administration:
For normal or immunocompromised adult patients with Herpes simplex infection, the recommended dosage is 5 mg/kg every 8 hours. For very severe Herpes zoster infection (shingles) with a normal immune system, the recommended dosage is 5 mg/kg every 8 hours, and for immune-compromised patients, the dosage recommendation is 10 mg/kg every 8 hours. For Herpes simplexencephalitis, the recommended dosage is 10 mg/kg every 8 hours. For neonates, the recommended dosage is 10-20 mg/kg every 8 hours. For children from 1 year to 12 years, the recommended dosage is 5 mg/kg every 8 hours for Herpes simplex infections and 10 mg/kg every 8 hours for Varicella zoster virus infection or with Herpes simplex encephalitis. Each dose should be administered by slow intravenous infusion over an one-hour period.
Preparation:
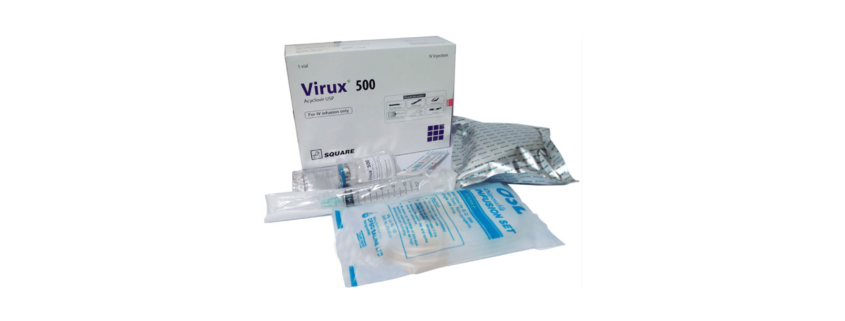
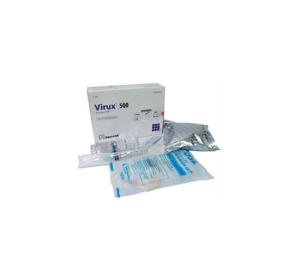
Virux® 500 IV Injection: Each combo pack contains one vial of Acyclovir 500 mg accompanied with 100 ml 0.9 % Sodium Chloride solution (SoloTM), one infusion set, one alcohol prep. pad, one first aid band & one 10 ml sterile disposable syringe.
Related Medicine: Xenole™, Xenole™, Vilanti™ Cozycap, Vonokit™, Virux Tablet®,Waxnil™