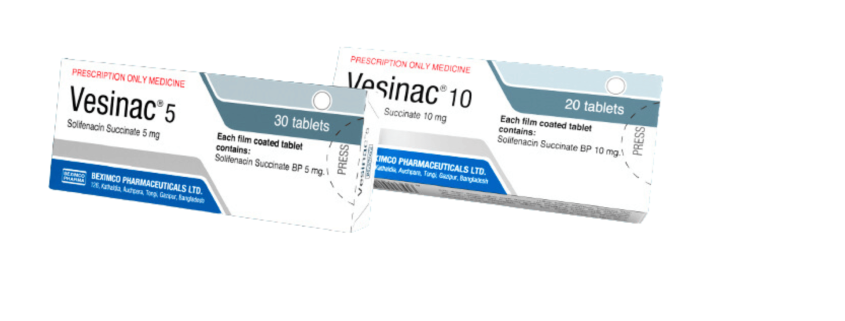
Vesinac
Generic Name: Solifenacin Succinate
Dosage Form: Tablet
TG Name: Urogenital
1. What is Vesinac®?
Vesinac® (Solifenacin Succinate) is a M3 selective muscarinic receptor antagonist approved by the FDA for the treatment of Overactive Bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. European Association of Urology (EAU), National Institute of Health and Care (NICE), American Urological Association (AUA) and National Health Service (NHS) all recommend that 1st line pharmacological treatment of OAB should be with anti-muscarinic agents such as Vesinac® (Solifenacin Succinate).
2. How Vesinac® works in our body?
Vesinac® (Solifenacin Succinate) is a M3 selective muscarinic receptor antagonist. It binds to the muscarinic receptors located in the detrusor muscles on the bladder wall. As the drug binds to the muscarinic receptors of the bladder smooth muscles (detrusor muscles), it reduces the bladder contractions thereby alleviating the OAB symptoms.
3. What is the indication of Vesinac®?
Vesinac® (Solifenacin Succinate) is a FDA approved M3 selective muscarinic receptor antagonist indicated for the treatment of Overactive Bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency.
4. What are the dosage & administration of Vesinac®?
Dosage & administration of Vesinac® for adults:
- 5 mg tablet taken once daily, and if well tolerated may be increased to 10 mg once daily.
- 5 mg Vesinac® tablet can be taken in combination with 25 mg Mirasol XR® (Mirabegron) tablet for better efficacy and tolerability by patients who does not respond well in monotherapy.
- 5 mg / 10 mg Vesinac® tablet can be taken in combination with 0.4 mg Uroflo® (Tamsulosin) capsules in case of BPH concurrent with LUTS.
- Do not exceed 5 mg tablet once daily in patients with:
- severe renal impairment [Creatinine Clearance] (CLCR<30 ml/min)
- moderate hepatic impairment (Child-Pugh B)
- concomitant use of potent CYP3A4 inhibitors
- Use of Vesinac® is not recommended in patients with severe hepatic impairment (Child-Pugh C).
5. What are the contraindications of Vesinac®?
Vesinac® is contraindicated in patients with:
- Hypersensitivity to the active substance or to any of the excipients
- Urinary retention
- Gastric retention
- Uncontrolled narrow-angle glaucoma
- Myasthenia gravis
- Patients undergoing hemodialysis
- Patients with severe hepatic impairment
- Patients with Congenital or Acquired QT Prolongation
6. What are the adverse effects of Vesinac®?
Adverse effects of Vesinac® are:
- Very common: Dry mouth
- Common: Blurred vision, constipation, nausea, dyspepsia, abdominal pain
- Uncommon: Urinary tract infection, cystitis, somnolence, dysgeusia, dry eyes, nasal dryness, gastro-esophageal reflux diseases, dry throat, dry skin, difficulty in micturition, fatigue peripheral edema
- Rare: Dizziness, headache, colonic obstruction, fecal impaction, vomiting, pruritus, rash, urinary retention
- Very rare: Hallucinations, confusional state, erythema multiforme, urticaria, angioedema
7. What would be the storage condition of Vesinac®?
Do not store above 30°C. Keep in a dry place. Protect from light & keep out of the reach of children.