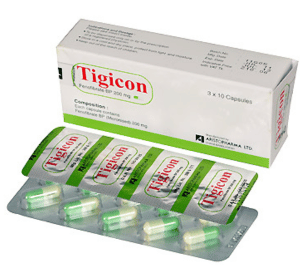
Tigicon (Fenofibrate)
Therapeutic Group : Lipid Lowering Agent
Presentation:
Tigicon Capsule: Each capsule contains Fenofibrate BP 200 mg.
Indications:
Tigicon is indicated for treatment of hyperlipidemia of Type IIa, IIb, III, IV and V in patients who have not responded adequately to diet & other appropriate measures.
Dosage & Administration:
The dose is one capsule per day. Fenofibrate capsule should be taken after meal. Renal insufficiency: The dosage of Fenofibrate should be minimized in patients who have severe renal impairment.
Contrainidications:
Patients with liver disease or gallstone, renal impairment & hypersensitivity to Fenofibrate.
Warning & Precautions:
Special care should be needed In patients with renal disease. Discontinuation of the drug is necessary if creatinine kinase concentration increases significantly.
Side effects:
Nausea, anorexia, gastric pain, pruritus, urticaria, headache, dizziness, vertigo, fatigue.
Drug interaction:
Fenofibrate potentiates the action of oral anticoagulants. In patients receiving oral anticoagulant therapy the dose should be reduced by one third on starting Fanofibrates. Fenofibrates should not be used in combination with perhexiline maleate or with monoamine oxidase inhibitors.
Use in special groups:
Use in ediatrics: Fenofibrate has not been investigated in adequate and well controlled trials in pediatric patients.
Use in pregnancy & nursing mothers: Fenofibrate is contraindicated during pregnancy & nursing mothers.
Packing:
Tigicon Capsule: Each box contains 30’s capsules in blister pack.