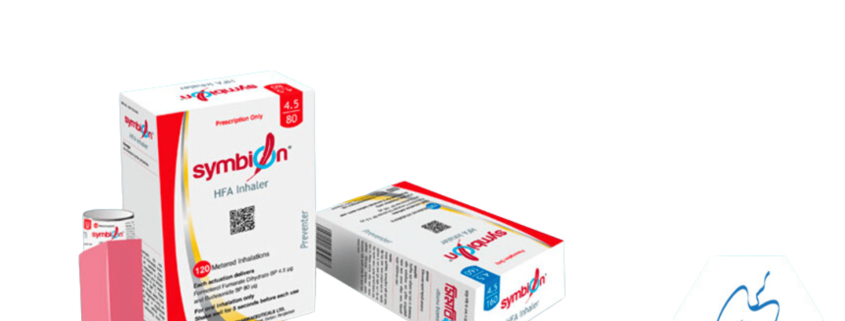
Symbion HFA

Generic Name: Formoterol fumarate dihydrate 4.5 µg & Budesonide 160 µg
Dosage Form: Inhaler
TG Name: Respiratory
What Symbion is and what it is used for?
Symbion® Bexicap capsule is a combination of Formoterol Fumarate Dihydrate and Budesonide.
Symbion® Bexicap is indicated in the regular treatment of asthma, where the use of a combination (long-acting, beta2-agonist and inhaled corticosteroid) has been found to be appropriate. It is also indicated in the symptomatic treatment of severe chronic obstructive pulmonary disease (COPD), with a history of repeated exacerbations despite regular therapy with long-acting bronchodilators.
How Symbion® HFA works?
Formoterol in Symbion® causes rapid bronchodilation by binding with beta-2 receptor in the airways. Budesonide binds with glucocorticoid receptor reducing inflammation in the airways.
What is the indication of Symbion® HFA Inhaler?
Symbion® HFA Inhaler (Formoterol Fumarate Dihydrate and Budesonide) is indicated for the long-term, twice-daily, maintenance treatment of asthma in patients 6 years of age and older where the use of a combination (long-acting, selective b2-agonist and inhaled corticosteroid) has been found to be appropriate.
What are the Dosage and Administration of Symbion® HFA Inhaler?
Asthma
Dosage is individual and should be adjusted according to disease severity. When control has been achieved, the dose should be titrated to the lowest effective dose, which could include Symbion® HFA Inhaler used once daily. For Symbion® HFA Inhaler there is two treatment approaches:
A. Maintenance Therapy: Symbion® is taken as regular maintenance treatment with a separate rapid acting bronchodilator as rescue. Patients should be advised to have their separate rapid acting bronchodilator available for rescue use at all times.
Adults (18 Years and Older)
Symbion® 4.5/80 HFA Inhaler or Symbion® 4.5/160 HFA Inhaler: 1-2 inhalations, twice daily
Adolescents (12-17 Years)
Symbion® 4.5/160 HFA Inhaler: 1-2 inhalations, twice daily
Children (6-11 Years)
Symbion® 4.5/80 HFA Inhaler: 1 inhalation, twice daily
For all patients it is desirable to titrate to the lowest effective strength after adequate asthma stability is achieved.
Improvement in asthma control following inhaled administration of Symbion® HFA Inhaler can occur within 15 minutes of beginning treatment, although maximum benefit may not be achieved for 2 weeks or longer after beginning treatment. Individual patients will experience a variable time to onset and degree of symptom relief.
B. Single Maintenance and Reliever Therapy: Symbion® is taken as regular maintenance and as needed in response to symptoms. Patients take daily maintenance dose of Symbion® HFA Inhaler and in combination take Symbion® HFA Inhaler as needed in response to symptoms. Patients should be advised to always have Symbion® HFA Inhaler available for use.
Adults (18 years and older): The recommended maintenance dosage is 2 inhalations per day as maintenance therapy (either one inhalation twice daily, or two inhalations in either the morning or the evening), although some patients may require two inhalations twice daily. Patients should take 1 additional inhalation as needed in response to symptoms. If symptoms persist after a few minutes, an additional inhalation should be taken. Not more than 6 inhalations should be taken on any single occasion.
What are the Warnings and Precautions of Symbion® HFA Inhaler?
It is recommended that the dose is tapered when the treatment is discontinued and should not be stopped abruptly. If patients find the treatment ineffective, or exceed the highest recommended dose of Symbion® HFA Inhaler, medical attention must be sought. Sudden and progressive deterioration in control of asthma is potentially life threatening and the patient should undergo urgent medical assessment.
What are the Side Effects of Symbion® HFA Inhaler?
Since Symbion® HFA Inhaler contains both Formoterol Fumarate Dihydrate and Budesonide, the same pattern of undesirable effects as reported for these substances may occur. No increased incidence of adverse reactions has been seen following concurrent administration of the two compounds.
Formoterol Fumarate Dihydrate: Most common adverse events with Formoterol are tremor, palpitations, headache etc. Some uncommon and rare adverse events that occurred in the groups receiving Formoterol are cardiac arrhythmias, muscle cramps, and hypersensitivity reactions such as rash, oedema and angio-oedema etc.
Budesonide: The incidence of common adverse events with Budesonide are oropharyngeal candidiasis, hoarseness or throat irritation, dysphonia, back pain, nausea, sinusitis, adrenal suppression, etc.
What would be the storage condition of Symbion® HFA Inhaler?
Pressurized canister, do not puncture, break or incinerate even when apparently empty. Avoid storage in direct sunlight or heat. Store below 30°C. Keep away from eyes. Keep away from children.
Discard within 3 months after removing from foil pouch.
Commercial Pack
Symbion® 4.5/80 HFA Inhaler: Each canister contains 120 metered doses, each actuation delivers Formoterol Fumarate Dihydrate BP 4.5 µg and Budesonide BP 80 µg.
Symbion® 4.5/160 HFA Inhaler: Each canister contains 120 metered doses, each actuation delivers Formoterol Fumarate Dihydrate BP 4.5 µg and Budesonide BP 160 µg.