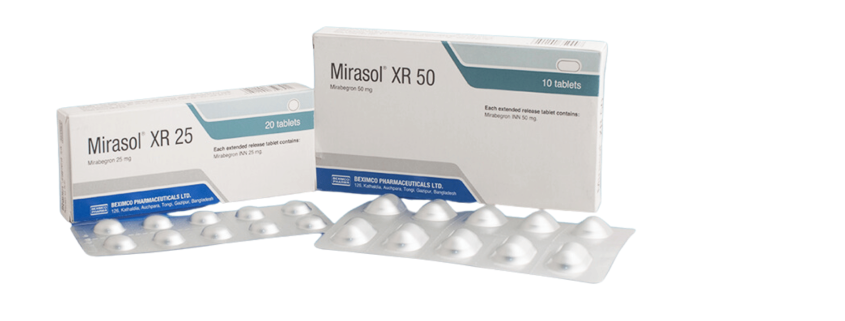
Mirasol XR
Generic Name: Mirabegron
Dosage Form: Tablet
TG Name: Urogenital
What Mirasol® XR is and what it is used for?
Mirasol® XR is a beta 3 adrenergic agonist approved by the FDA to treat overactive bladder (OAB) with symptoms of urgency, frequency and leakage in adults. Mirasol® is the first and only drug in its class for OAB. Mirasol® XR is indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency and urinary frequency.Mirasol® XR in combination with the muscarinic antagonist solifenacin succinate is indicated for the treatment of OAB with symptoms of urge urinary incontinence, urgency and urinary frequency.
Before you take Mirasol® XR
Do not use Mirasol® XR if hypersensitivity reactions to mirabegron or tablet components have occurred.
How to take Mirasol® XR
Recommended starting dose is 25mg once daily alone or in combination with solifenacin succinate 5mg once daily. Based on individual efficacy and tolerability, may increase dose to 50mg once daily, alone or in combination with solifenacin succinate 5mg once daily. Swallow whole with water or without food, do not chew, divide or crush. Patients with severe renal impairment or patients with moderate hepatic impairment: Maximum dose is 25mg Mirasol® XR once daily. Patients with end stage renal disease (ESRD) or patients with severe hepatic impairment: Not recommended.
Possible side effects
Increased blood pressure, Headache, Cold symptoms such as stuffy nose, Sneezing, Sore throat, Constipation, Diarrhea.
Pregnancy and Lactation
There are no adequate and well-controlled studies using Mirasol XR in pregnant women. Mirasol XR should be used during pregnancy only if the potential benefit to the patient outweighs the risk to the patient and fetus. Women who become pregnant during Mirasol XR treatment are encouraged to contact their physician.
Warnings & Precaution
Mirasol® XR alone or in combination with solifenacin succinate 5mg can increased blood pressure. Periodic blood pressure determinations are recommended, especially in hypertensive patients. Mirasol® is not recommended for use in severe uncontrolled hypertensive patients. Urinary retention in patients with bladder outlet obstruction and in patients taking muscarinic antagonist drugs for overactive bladder: Administer with caution in these patients because of risk of urinary retention. Angioedema: Angioedema of the face, lips, tounge and or larynx has been reported with Mirasol®. Patients taking drugs metabolized by CYP2D6: Mirasol® XR is a moderate inhibitor of CYP2D6. Appropriate monitoring is recommended and dose adjustment may be necessary for narrow therapeutic index CYP2D6 substrates.
How to store Mirasol® XR
Keep in a dry place and store below 30 degree C. Protect from light and keep out of the reach of children.