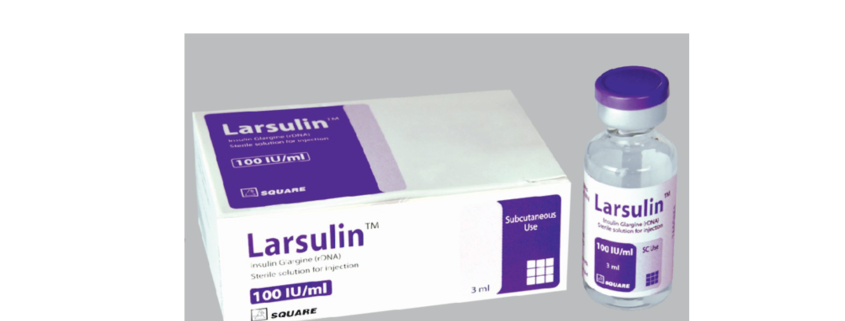
Larsulin™
Insulin Glargine
Analog Insulin (Antidiabetic Preparations)
Indication:
Type 1 and Type 2 Diabetes Mellitus.
Dosage & Administration:
Larsulin™ exhibits a relatively constant glucose-lowering profile over 24 hours that permits once-daily dosing. Potency of insulin glargine is approximately the same as human insulin.
Larsulin™ is recommended for once daily subcutaneous administration & may be administered at any time during the day. However, once started should be administered at the same time every day.
The dose of Larsulin™ must be individualized based on clinical response. Blood glucose monitoring is essential in all patients with diabetes. In patients with type 1 diabetes, Larsulin™ must be used in regimens with short-acting insulin. Larsulin™ is not recommended for intravenous administration.
Intravenous administration of the usual subcutaneous dose could result in severe hypoglycemia.
Initiation of Larsulin™ therapy:
The recommended starting dose of Larsulin™ in patients with type 1 diabetes should be approximately one-third of the total daily insulin requirements. Short-acting, premeal insulin should be used to satisfy the remainder of the daily insulin requirements.
The recommended starting dose of Larsulin™ in patients with type 2 diabetes who are not currently treated with insulin is 10 units (or 0.2 Units/kg) once daily, which should subsequently be adjusted to the patient’s needs.
Converting to Larsulin™ from other insulin therapies:
If changing from a treatment regimen with an intermediate-or long-acting insulin to a regimen with Larsulin™ , the amount and timing of shorter-acting insulins and doses of any oral anti-diabetic drugs may need to be adjusted.
If transferring patients from once-daily NPH insulin to once-daily Larsulin™, the recommended initial Larsulin™ dose is the same as the dose of NPH that is being discontinued.
If transferring patients from twice-daily NPH insulin to once-daily Larsulin™, the recommended initial Larsulin™ dose is 80% of the total NPH dose that is being discontinued.
Preparation:
Larsulin™ Injection: Each box contains 3 ml glass vial.
Larsulin™ Pen Cartridge: Each box contains 3 ml glass Cartridge.