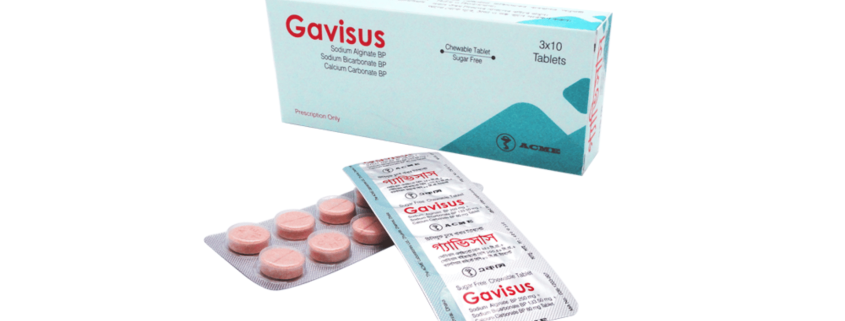
Gavisus
Sodium Alginate, Sodium Bicarbonate & Calcium Carbonate
GASTROINTESTINAL
INDICATION
Gastric reflux, heartburn, flatulence associated with gastric reflux, heartburn of pregnancy, all cases of epigastric and retrosternal distress where the underlying cause is gastric reflux.
DOSAGE AND ADMINISTRATION
Route of administration: Oral.
Gavisus Suspension:
Adult and children over 12 years: 10-20 ml after meals and at bedtime, up to four times a day.
Children 6 to 12 years: 5-10 ml after meals and at bedtime, up to four times a day.
Children under 6 years: Not recommended.
Elderly: No dosage modification is required for this age group.
Gavisus DX Suspension:
Adults and children 12 years and over: 10-20 ml after meals and at bedtime, up to four times per day.
Children under 12 years: Should be given only on medical advice.
Elderly: No dose modifications necessary for this age group.
Hepatic Impairment: No dose modification necessary.
Renal Insufficiency: Caution if highly restricted salt diet is necessary.
Gavisus Tablet:
Adult and children 12 years and over: 2-4 tablets after meals and at bedtime, up to four times per day.
Children under 12 years: Should be given only on medical advice.
Elderly: No dosage modification is required for this age group.
Hepatic Impairment: No dosage modification necessary.
Renal Insufficiency: Caution if highly restricted salt diet is necessary.
COMPOSITION
Gavisus Suspension: Each 10 ml suspension contains Sodium Alginate BP 500 mg, Sodium Bicarbonate BP 267 mg & Calcium Carbonate BP 160 mg.
Gavisus DX Suspension: Each 10 ml suspension contains Sodium Alginate BP 500 mg, Sodium Bicarbonate BP 213 mg & Calcium Carbonate BP 325 mg.
Gavisus Chewable Tablet: Each chewable tablet contains Sodium Alginate BP 250 mg, Sodium Bicarbonate BP 133.50 mg & Calcium Carbonate BP 80 mg.