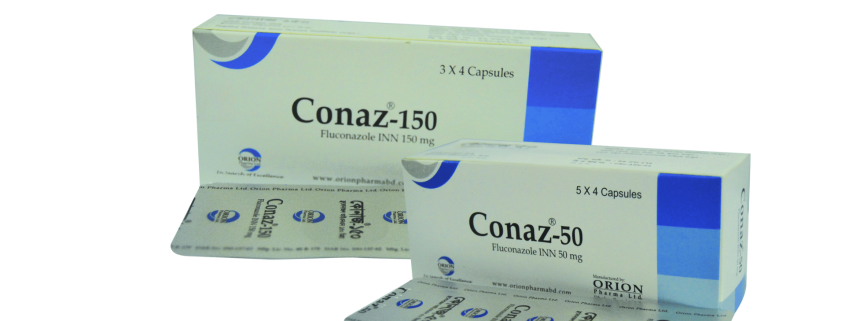
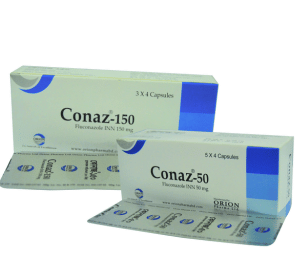
Conaz
ANTIFUNGALS: Fluconazole
Indication
It is indicated in the treatment of vaginal candidiasis and candidal balanitis, mucosal condidiasis (as oropharyngeal candidiasis, atrophic oral candidiasis, oesophagitis, candiduria); tinea pedis, corporis, cruris, pityriasis versicolor and dermal condidiasis; candidaemia and disseminated candidiasis and cryptococcal infections (including meningitis).
Contraindication
Fluconazole is contraindicated in patients with known hypersensitivity to the drug. Fluconazole therapy should be discontinued if signs and symptoms consistent with liver disease develop.
Dosage & Administration
Vaginal candidiasis & candidal balanitis-a single dose of 150 mg. Mucosal candidiasis (except genital) – 50 mg daily (100 mg daily in unusually difficult infections) given for 7-14 days in oropharyngeal candidiasis; for 14 days in atrophic oral candidiasis associated with dentures; for 14- 30 days in other mucosal infections (eg. oesophagitis, candiduria). Child, 3-6 mg/ kg on first day then 3 mg / kg daily.
Tinea pedis, corporis, cruris, pityriasis, versicolor and dermal candidiasis- 50 mg daily for 2- 4 weeks (up to 6 weeks in tinea pedis).
Invasive candidial infections (including candidaemia and disseminated candidiasis) and cryptococcal infections (including meningitis), 400 mg initially then 200 mg daily, increased if necessary to 400 mg daily; treatment continued according to response (at least 6-8 weeks for cryptococcal meningitis); child 6-12 mg/daily (every 72 hours in neonate up to 2 weeks old); maximum 400 mg daily.
Prevention of relapse of cryptococcal meningitis in AIDS patients after completion of primary therapy, 100-200 mg daily.
Prevention of fungal infections in immunocompromised patients following cytotoxic chemotherapy or radiotherapy, 50-400 mg daily adjusted according to risk.
Side Effect
Nausea, abdominal discomfort, diarrhoea and flatulence; occassionally abnormalities of liver enzymes; rarely rash, angioedema; anaphylaxis reported.
Precaution
Caution should be taken in renal impairment, pregnancy and breast feeding and in raised liver enzymes. The use of fluconazole in nursing mother is not recommended.
Drug Interaction
Rifampicin reduces plasma concentration of Fluconazole. Effect of nicoumalone and warfarin is enhanced. Plasma concentrations of sulphonylureas & theophylline is possibly increased. Effect of phenytoin is enhanced.
Presentation
Conaz 50 capsule : Box containing 5 x 4 capsules in blister pack.
Conaz 150 capsule : Box containing 3 x 4 capsules in blister pack.