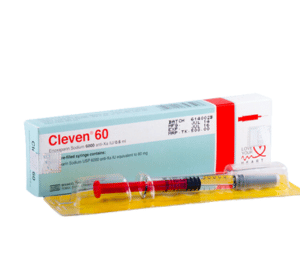
Cleven
Generic Name: Enoxaparin
Dosage Form: Sodium
TG Name: Cardiovascular
What is CLEVEN®?
Cleven is a READY-TO-USE sterile aqueous solution containing enoxaparin sodium, a low molecular weight heparin with a high anti-Xa activity and with a low anti-lla or anti- thrombin activity. Enoxaparin sodium is obtained by alkaline depolymerization of heparin benzyl ester derived from porcine intestinal mucosa. At doses required for the various indications, Enoxaparin does not increase bleeding time. At preventive doses, Enoxaparin causes no notable modification of activated Partial Thromboplastin Time (aPTT). It neither influences platelet aggregation nor binding of fibrinogen to platelets. Enoxaparin is primarily metabolized in the liver.
What are the available strengths of CLEVEN®?
CLEVEN 20: Each Pre-filled syringe contains Enoxaparin sodium 20 mg (equivalent to 2,000 anti-Xa IU) in 0.2 mL Water for Injection.
CLEVEN 40: Each Pre-filled syringe contains Enoxaparin sodium 40 mg (equivalent to 4,000 anti-Xa IU) in 0.4 mL Water for Injection.
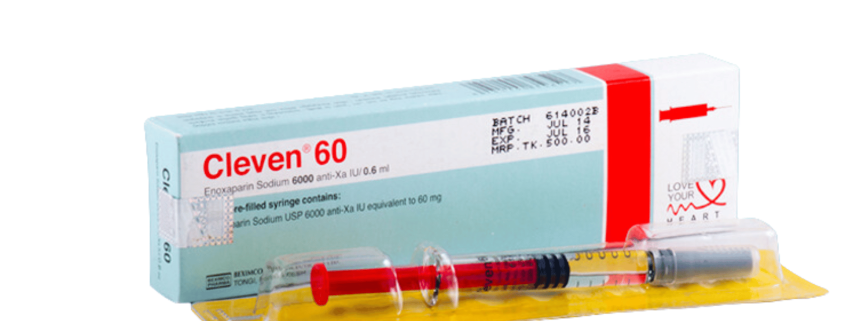
CLEVEN 60: Each Pre-filled syringe contains Enoxaparin sodium 60 mg (equivalent to 6,000 anti-Xa IU) in 0.6 mL Water for Injection.
CLEVEN 80: Each Pre-filled syringe contains Enoxaparin sodium 80 mg (equivalent to 8,000 anti-Xa IU) in 0.8 mL Water for Injection
What are the indications of CLEVEN?
Cleven is indicated for:
- Treatment of venous thromboembolic disease presenting with deep vein thrombosis, pulmonary embolism or both.
- Treatment of unstable angina and non-Q-wave myocardial infarction, administered concurrently with aspirin.
- Treatment of acute ST-segment Elevation Myocardial Infarction (STEMI) including patients to be managed medically or with subsequent Percutaneous Coronary Intervention (PCI) in conjunction with thrombolytic drugs (fibrin or non-fibrin specific).
- Prophylaxis of thromboembolic disorders of venous origin, in particular those which may be associated with orthopaedic or general surgery.
- Prophylaxis of venous thromboembolism in medical patients bedridden due to acute illness.
- Prevention of thrombus formation in the extracorporeal circulation during haemodialysis.
What are the dosage & administration of CLEVEN?
Adults: Prophylaxis of venous thromboembolism: In patients with a low to moderate risk of venous thromboembolism the recommended dosage is 20 mg (2,000 IU) once daily by subcutaneous injection for 7 to 10 days, or until the risk of thromboembolism has diminished. In patients undergoing surgery, the initial dose should be given approximately 2 hours pre-operatively. In patients with a higher risk, such as in orthopaedic surgery, the dosage should be 40 mg (4,000 IU) daily by subcutaneous injection with the initial dose administered approximately 12 hours before surgery.
Prophylaxis of venous thromboembolism in medical patients: The recommended dose of enoxaparin sodium is 40 mg (4,000 IU) once daily by subcutaneous injection. Treatment with enoxaparin sodium is prescribed for a minimum of 6 days and continued until the return to full ambulation, for a maximum of 14 days.
Treatment of venous thromboembolism: Cleven should be administered subcutaneously as a single daily injection of 1.5 mg/kg (150 IU/kg). Cleven treatment is usually prescribed for at least 5 days and until adequate oral anticoagulation is established.
Treatment of unstable angina and non-Q-wave myocardial infarction: The recommended dose is 1 mg/kg Cleven every 12 hours by subcutaneous injection, administered concurrently with oral aspirin (100 to 325mg once daily). Treatment with Cleven in these patients should be prescribed for a minimum of 2 days and continued until clinical stabilisation. The usual duration of treatment is 2 to 8 days. Treatment of acute ST-segment Elevation Myocardial Infarction: The recommended dose of enoxaparin sodium is a single IV bolus of 30mg plus a 1mg/kg SC dose followed by 1mg/kg administered SC every 12 hours (max 100mg for the first two doses only, followed by 1mg/kg dosing for the remaining doses).
Prevention of extracorporeal thrombus formation during haemodialysis: A dose equivalent to 1 mg/kg (100 IU/kg) introduced into the arterial line at the beginning of a dialysis session is usually sufficient for a 4 hour session. If fibrin rings are found, such as after a longer than normal session, a further dose of 0.5 to 1mg/kg (50 to 100 IU/kg) may be given. For patients at a high risk of haemorrhage the dose should be reduced to 0.5 mg/kg (50 IU/kg) for double vascular access or 0.75 mg/kg (75 IU/kg) for single vascular access.
Elderly: For treatment of acute ST-segment Elevation Myocardial Infarction in elderly patients 75 years of age, do not use an initial IV bolus. Initiate dosing with 0.75mg/kg SC every 12 hours (maximum 75mg for the first two doses only, followed by 0.75mg/kg dosing for the remaining doses).For other indications, no dosage adjustments are necessary in the elderly, unless kidney function is impaired.
Children: Not recommended, as dosage not established.
Renal impairment: Severe renal impairment: A dosage adjustment is required for patients with severe renal impairment (creatinine clearance < 30 ml/min), according to the following tables, since enoxaparin sodium exposure is significantly increased in this patient population:
Dosage adjustments for therapeutic dosage ranges
| Title | Awareness |
| Standard dosing | Severe renal impairment |
| 1 mg/kg SC twice daily | 1 mg/kg SC once daily |
| 1.5 mg/kg SC once daily | 1 mg/kg SC once daily |
| 30mg-single IV bolus plus a 1mg/kg SC dose followed by 1mg/kg twice daily. | 30mg-single IV bolus plus a 1mg/kg SC dose followed by 1mg/kg once daily. |
| Elderly patients GREATER-THAN OR EQUAL TO (8805) 75 years of age (for acute STEMI indication only) | |
| 0.75mg/kg SC twice daily without initial bolus. | 1mg/kg SC once daily without initial bolus. |
Dosage adjustments for prophylactic dosage ranges
| Standard dosing | Severe renal impairment |
| 40 mg SC once daily | 20 mg SC once daily |
| 20 mg SC once daily | 20 mg SC once daily |
Moderate and mild renal impairment:
Although no dosage adjustments are recommended in patients with moderate renal impairment (creatinine clearance 30-50 ml/min) or mild renal impairment (creatinine clearance 50-80 ml/min), careful clinical monitoring is advised.
Hepatic impairment: Caution should be exercised.
Body weight: No dosage adjustments are recommended in obesity or low body weight.
What are the warning & precautions of CLEVEN?
Enoxaparin injection should not be administered by intramuscular route. Enoxaparin should be used with caution in conditions with increased potential for bleeding, such as impaired hemostasis, history of peptic ulcer, recent ischemic stroke, uncontrolled severe arterial hypertension, diabetic retinopathy, recent neuro or opthalmologic surgery and low weight patients (low-weight women (< 45 kg) and low-weight men (< 57 kg). It is recommended that the platelet count be measured befored the initiation of the treatment and regularly thereafter during treatment.
What are the possible drug-drug interactions of CLEVEN?
It is recommended that agents which affect haemostasis should be discontinued prior to enoxaparin therapy unless their use is essential, such as: systemic salicylates, acetylsalicylic acid, NSAIDs including ketorolac, dextran, and clopidogrel, systemic glucocorticoids, thrombolytics and anticoagulants. If the combination cannot be avoided, enoxaparin should be used with careful clinical and laboratory monitoring.
What are the adverse effects of CLEVEN?
Haemorrhage (bleeding), Thrombocytopenia, elevations of serum aminotransferase. Pain, hepatic enzymes increase (mainly transaminases), urticaria, pruritus, erythema, bluish marks at injection sites to skin rash at injection sites. Cases of neuraxial hematomas with the concurrent use of Enoxaparin and spinal/epidural anesthesia or spinal puncture have resulted in varying degrees of neurologic injuries.
What would be the storage condition of CLEVEN?
Enoxaparin should be stored below 25° C. Do not freeze. Do not store the multiple-dose vials for more than 28 days after the first use. Keep out of the reach of children.