Axon (Ceftriaxone)
Description
Axon is a 3rd generation broad-spectrum parenteral cephalosporin antibiotic. It has potent bactericidal activity against a wide range of Gram-positive and Gram-negative organisms. Axon like other cephalosporins and penicillins kills bacteria by interfering with the synthesis of the bacterial cell wall. Axon has a high degree of stability in the presence of beta lactamases. A remarkable feature of Axon is its relatively long plasma elimination half-life of about 6 to 9 hours, which makes single or once-daily dosage of the drug appropriate for most patients. Ceftriaxone is not metabolized in the body. About 40-65% of a dose of Ceftriaxone is excreted unchanged in the urine; the remainder is excreted in the bile and ultimately found in the feces as unchanged drug and microbiologically inactive compound. The drug is highly protein bound (95%).
Presentation
Axon 250 mg IM/IV Injection: Each vial contains dry powder equivalent to 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 2 ml 1% Lidocaine Injection USP for IM injection or 5 ml Water for Injection BP for IV injection.
Axon 500 mg IM/IV Injection: Each vial contains dry powder equivalent to 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 2 ml 1% Lidocaine Injection USP for IM injection or 5 ml Water for Injection BP for IV injection.
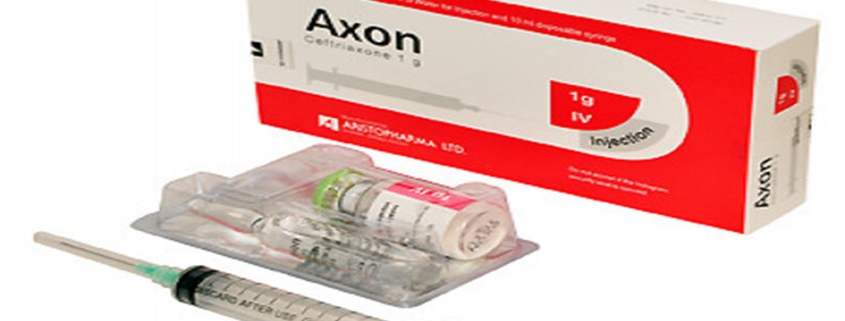
Axon 1 g IM/IV Injection: Each vial contains dry powder equivalent to 1 g Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 3.5 ml Lidocaine 1% Injection USP for IM injection or 10 ml Water for Injection BP for IV injection.
Axon 2 g IV Injection: Each vial contains dry powder equivalent to 2 g Ceftriaxone ( as sterile Ceftriaxone Sodium USP) and 2 ampoules of 10 ml Water for Injection BP for IV injection.
Indications
Axon is indicated for the treatment of the following major infections:
- Renal and urinary tract infections
- Lower respiratory tract infections, particularly pneumonia
- Gonococcal infections
- Skin,soft tissue, bone and joint infections
- Bacterial meningitis
- Serious bacterial infections e.g. septicemia
- ENT infections
- Infections in cancer patients
- Prevention of postoperative infections
- Perioperative prophylaxis of infections associated with surgery
- Typhoid fever.
Dosage & Administration
Adult: By deep intramuscular injection, or by intravenous injection over at least 2-4 minutes, or by intravenous infusion, 1 g daily; 2-4 g daily in severe infections; intramuscular doses over 1 g divided between more than one site. Neonate: By intravenous infusion over 60 minutes, 20-50 mg/kg daily (max.50 mg/kg daily). Infant and child under 50 kg: by deep intramuscular injection or by intravenous injection over 2-4 minutes or by intravenous infusion, 20-50 mg/kg daily; up to 80 mg/kg daily in severe infections; doses of 50 mg/kg and over by intravenous infusion only; 50 kg and over, adult dose.
Uncomplicated gonorrhea: by deep intramuscular injection, 250 mg as a single dose. Surgical prophylaxis: by deep intramuscular injection or by intravenous injection over at least 2-4 minutes, 1 g at induction, Colorectal surgery: by deep intramuscular injection or by intravenous injection over at least 2-4 minutes or by intravenous infusion, 2 g at induction; intramuscular doses over 1 g divided between more than one site.
Duration of therapy: Continue for more than 2 days after signs and symptoms of infection have disappeared. Usual duration is 4 to 14 days; in complicated infections, longer therapy may be required.
Preparation of Solutions for Intramuscular/intravenous Injections:
For intramuscular injection: 250 mg or 500 mg Axon should be dissolved in 2 ml of 1% Lidocaine Injection USP or 1 g Axon in 3.5 ml of 1% Lidocaine Injection USP. For intravenous injection: 250 mg or 500 mg Axon should be dissolved in 5 ml of Water for Injection BP or 1 g Axon in 10 ml of Water for Injection BP or 2 g Axon in 20 ml of Water for Injection BP. The injection should be administered over 2-4 minutes, directly into the vein or via the tubing of an intravenous infusion. The use of freshly reconstituted solution is recommended. However, it maintains potency for at least 6 hours at room temperature or 24 hours at 5°C.
Contrainidications
Ceftriaxone should not be given to patients with a history of hypersensitivity to cephalosporin antibiotics.
Warning & Precautions
The admixture of beta-lactam antibacterials (penicillins and cephalosporins) and aminoglycosides may result in substantial mutual inactivation. If they are administered concurrently , they should be administered in separate sites. Do not mix them in the same intravenous bag or bottle.
Side effects
Ceftriaxone is generally well tolerated. A few side effects such as gastro-intestinal effects including diarrhoea, nausea and vomiting, stomatitis & glossitis; cutaneous reactions including rash, pruritus, urticaria, oedema & erythema multiforme; hematologic reactions including eosinophilia, thrombocytopenia, leucopenia, anemia and neutropenia; hepatic reactions including elevations of SGOT or SGPT, bilirubinemia; CNS reactions including headache, hyperactivity, nervousness, sleep disturbances, confusion, hypertonia and dizziness were reported. Local phlebitis occurs rarely following intravenous administration but can be minimized by slow injections over 2-4 minutes.
Drug interaction
No drug interactions have been observed with diuretics or with aminoglycosides.
Use in special groups
Use in pregnancy: Its safety in human pregnancy has not been established. Therefore it should not be used in pregnancy unless absolutely indicated.
Use in nursing mother: Only minimal amount of Ceftriaxone is excreted in breast milk, so mother-receiving Ceftriaxone should not breast-feed. In severe renal impairment accompanied by hepatic insufficiency, dosage reduction is required.
Packing
Axon 250 mg IM Injection: Combipack of 1 vial containing 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 2 ml 1% Lidocaine Injection USP.
Axon 250 mg IV Injection: Combipack of 1 vial containing 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 5 ml Water for Injection BP.
Axon 500 mg IM Injection: Combipack of 1 vial containing 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 2 ml 1% Lidocaine Injection USP.
Axon 500 mg IV Injection: Combipack of 1 vial containing 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 5 ml Water for Injection BP.
Axon 1g IM Injection: Combipack of 1 vial containing 1g Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 3.5 ml 1% Lidocaine Injection USP.
Axon 1g IV Injection: Combipack of 1 vial containing 1g Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 1 ampoule of 10 ml Water for Injection BP.
Axon 2g IV Injection: Combipack of 1 vial containing 2 g Ceftriaxone (as sterile Ceftriaxone Sodium USP) and 2 ampoules of 10 ml Water for Injection BP.