Acyvir Injection (Acyclovir)
Acyclovir is a synthetic purine nucleoside analogue which is active against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV).
Description
Acyclovir is a synthetic purine nucleoside analogue which is active against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. Acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in three ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase.
Presentation
Acyvir 250 mg Lyophilized Powder for Solution for Infusion: Each vial contains Acyclovir Sodium equivalent to Acyclovir USP 250 mg.
Acyvir 500 mg Lyophilized Powder for Solution for Infusion: Each vial contains Acyclovir Sodium equivalent to Acyclovir USP 500 mg.
Indications
- Herpes Simplex infections in immunocompromised patients
- Initial and recurrent mucosal and cutaneous Herpes Simplex (HSV-1 and HSV-2) infections in immunocompromised patients
- Severe initial clinical episodes of herpes genitalis in immunocompetent patients
- Herpes Simplex encephalitis
- Neonatal herpes simplex virus infections and varicella-zoster (shingles) infections in immunocompromised patients.
Dosage & Administration
Method of Preparation:
The required dose of Acyclovir IV for Infusion should be administered by slow intravenous infusion over a one-hour period. Acyclovir 250 mg IV for Infusion should be reconstituted in 10 ml of Water for Injections BP or Sodium Chloride Injection BP (0.9% w/v) and Acyclovir 500 mg IV for Infusion should be reconstituted in 10 ml of Water for Injections BP or Sodium Chloride Injection BP (0.9% w/v) to provide a solution containing 25/50 mg acyclovir per ml. From the calculated dose, determine the appropriate number of vials to be used. Shake the vial gently until the contents of the vial have dissolved completely. After reconstitution Acyclovir IV for Infusion may be administered by a controlled rate infusion pump. Alternatively, the reconstituted solution may be further diluted to give an acyclovir concentration of not greater than 5 mg/ml (0.5% w/v) for administration by infusion:- Add the required volume of reconstituted solution to Sodium Chloride 0.9% w/v infusion solution and shake well to ensure adequate mixing occurs.
- For children and neonates, where it is advisable to keep the volume of infusion fluid to a minimum, it is recommended that dilution is on the basis of 4 ml reconstituted solution of Acyvir 250 mg Injection or 2 ml reconstituted solution of Acyvir 500 mg Injection (100 mg Acyclovir) added to 20 ml of infusion fluid.
- For adults, it is recommended that infusion bags containing 100 ml of infusion fluid are used, even when this would give an Acyclovir concentration substantially below 0.5% w/v. Thus one 100 ml infusion bag may be used for any dose between 250 mg and 500 mg Acyclovir but a second bag must be used for doses between 500 mg and 1000 mg. When diluted in accordance with the recommended schedules, Acyclovir IV for Infusion is stable for up to 12 hours at room temperature. Reconstituted or diluted solution should not be refrigerated.
| Disease | Patient Group | Dosage | Duration |
| Herpes simplex encephalitis | Adults and Adolescents(12 years of age and older) | 10 mg/kg every 8 hours | 10 days |
| Pediatrics(3 months to 12 years of age) | 20 mg/kg every 8 hours | 10 days | |
| Herpes simplex infections | Adults and Adolescents(12 years of age and older) | 5 mg/kg every 8 hours | 7 days |
| Pediatrics(Under 12 years of age) | 10 mg/kg every 8 hours | 7 days | |
| Severe initial clinical episodes of Herpes genitalis | Adults and Adolescents(12 years of age and older) | 5 mg/kg every 8 hours | 5 days |
| Varicella zoster infections(Chickenpox) | Adults and Adolescents(12 years of age and older) | 10 mg/kg every 8 hours | 7 days |
| Pediatrics(Under 12 years of age) | 20 mg/kg every 8 hours | 7 days | |
| Neonatal Herpes simplex infections | Neonatal Herpes Simplex Virus Infections(Birth to 3 months) | 10 mg/kg every 8 hours | 10 days |
Warning & Precautions
Precipitation of acyclovir crystals in renal tubules can occur if the maximum solubility of free acyclovir is exceeded or if the drug is administered by bolus injection. When dosage adjustments are required they should be based on estimated creatinine clearance.
Side effects
Most common side effects are headache, nausea, diarrhea, rash and phlebitis.
Drug interaction
Coadministration of probenecid with acyclovir has been shown to increase the mean acyclovir half-life and the area under the concentration-time curve. Urinary excretion and renal clearance were correspondingly reduced.
Use in special groups
Use in Pregnancy: Pregnancy category B. There are no adequate and well-controlled studies in pregnant women. Acyclovir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Use in nursing mothers: Acyclovir should be administered to a nursing mother with caution and only when indicated. Geriatric Use: No overall differences in safety are observed between older and younger subjects.
Packing
Acyvir 250 mg Lyophilized Powder for Solution for Infusion: Each combipack contains 1 vial of 250 mg Acyclovir Lyophilized Powder (as Acyclovir Sodium) for solution for infusion, 1 ampoule of 10 ml water for injections BP & 10 ml disposable syringe.
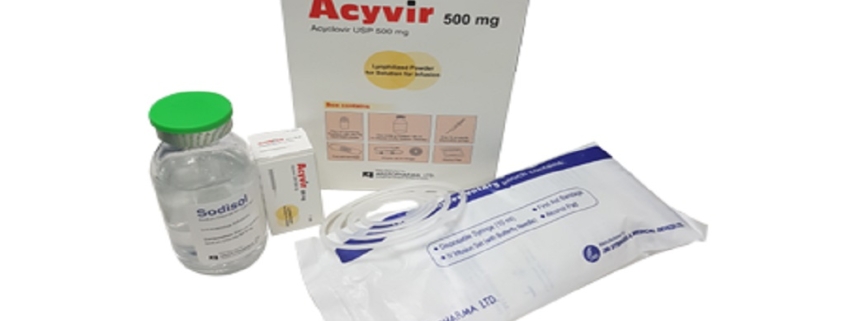
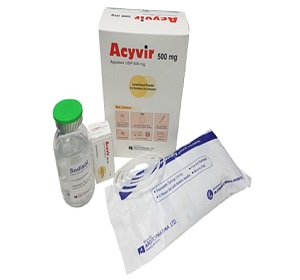
Acyvir 500 mg Lyophilized Powder for Solution for Infusion: Each combipack contains 1 vial of 500 mg Acyclovir Lyophilized Powder (as Acyclovir Sodium) for solution for infusion, 1/2 ampoules of 10 ml water for injections BP & 10 ml disposable syringe.