Bemsivir
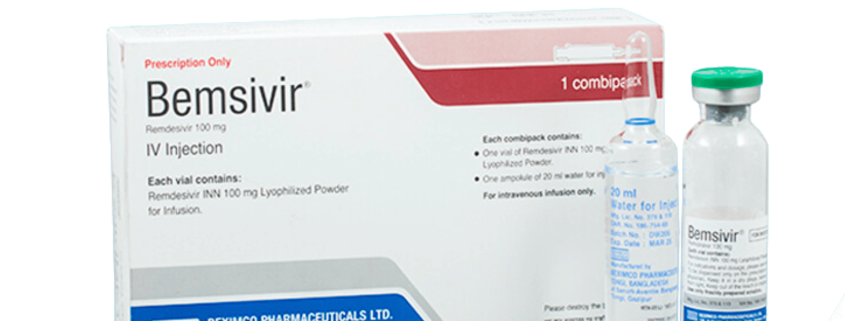
Generic Name: Remdesivir 100 mg, Lyophilized Powder
Dosage Form: IV INJECTION
TG Name: Antiviral
1. What BEMSIVIR is and what it is used for
Bemsivir is the preparation of remdesivir as lyophilized powder for IV injection. Remdesivir is an investigational nucleotide analog with broad-spectrum antiviral activity.
Uses:
Remdesivir is authorized for use under an Emergency Use Authorization (EUA) for treatment of patients hospitalized with suspected or laboratory confirmed SARS-CoV-2 infection and severe disease. Severe disease is defined as patients with an oxygen saturation (SpO2) ≤94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). Specifically, remdesivir is only authorized for hospitalized adult and pediatric patients for whom use of an intravenous agent is clinically appropriate.
2. What special warnings and precautions for use
- Hepatic laboratory testing should be performed in all patients prior to starting remdesivir and daily while receiving remdesivir.
- Remdesivir should not be initiated in patients with ALT ≥ 5 times the upper limit of normal at baseline.
3. Possible side effects
Remdesivir is still under investigation. So the side effects are not well known. However, as per the FDA, two possible side effects of remdesivir are as follows:
- Increase in the levels of liver enzymes, indicating that it may negatively affect the liver. Before giving remdesivir to a patient, doctors check their liver health through various blood tests.
- Infusion-related reaction during the administration of the drug. It shows up as nausea, vomiting, shivering, sweating and low blood pressure.
4. Special population:
Renal impairment
No dose adjustment of remdesivir is required for patients with mild and moderate renal impairment.
Patients with renal failure (eGRF < 30 mL/min) or dialysis or continuous veno-venous hemofiltration must not receive remdesivir.
Hepatic impairment
No dose adjustment of remdesivir is required for patients with mild and moderate hepatic impairment.
Patients with hepatic impairment (ALT > 5 x upper limit of normal (ULN)) must not receive remdesivir.
Elderly
No dose adjustment of remdesivir is required for elderly patients.
Paediatric population
The safety and effectiveness of remdesivir for treatment of COVID-19 have not been assessed in pediatric patients.
5. What BEMSIVIR dose is and how to take it
The optimal duration of treatment for COVID-19 is unknown. Under this EUA for remdesivir to treat COVID-19:
- The suggested dose for adults and pediatric patients weighing ≥40 kg requiring invasive mechanical ventilation and/or ECMO is a single loading dose of 200 mg infused intravenously over 30 to 120 minutes on Day 1 followed by once-daily maintenance doses of 100 mg infused intravenously over 30 to 120 minutes for 9 days (days 2 through 10).
- The suggested dose for adults and pediatric patients weighing ≥40 kg not requiring invasive mechanical ventilation and/or ECMO is a single dose of 200 mg infused intravenously over 30 to 120 minutes on Day 1 followed by once-daily maintenance doses of 100 mg infused intravenously over 30 to 120 minutes for 4 days (days 2 through 5). If a patient does not demonstrate clinical improvement, treatment may be extended for up to 5 additional days (i.e., up to a total of 10 days).
- Remdesivir should be administered via intravenous (IV) infusion only. Do not administer as an intramuscular (IM) injection.
6. How BEMSIVIR is supplied
Each box containing one vial of Remdesivir INN 100 mg lyophilized powder for infusion and one ampoule of 20 ml Water for Injection BP.