Wandara(Ertapenem for Injection)
Therapeutic Group: Antibiotic
Presentation
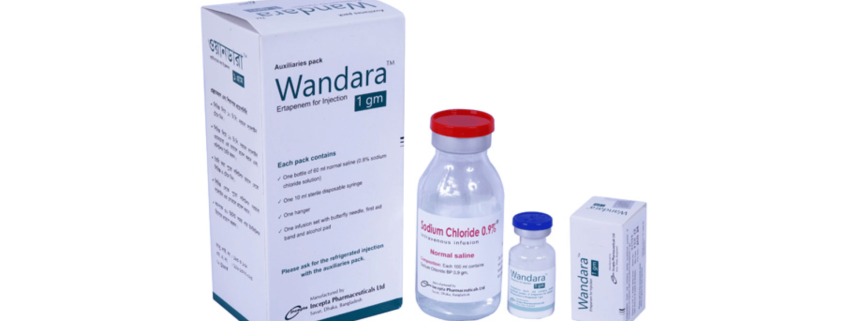
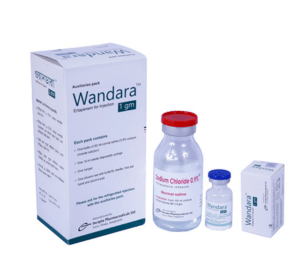
Wandara TM : Each vial contains sterile lyophilized powder of Ertapenem for Injection INN equivalent to Ertapenem 1 gm.
Description
Ertapenem for Injection is a sterile, synthetic, long-acting, parenteral, 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics, such as penicillins and cephalosporins, with activity against a wide range of Gram-positive and Gram negative aerobic and anaerobic bacteria.
Indications
Treatment: Ertapenem is indicated for the treatment of patients with moderate to severe infections caused by susceptible strains of microorganisms, as well as initial empiric therapy prior to the identification of causative organisms in the infections listed below:
• Complicated Intra-Abdominal Infections
• Complicated Skin and Skin Structure Infections including diabetic lower extremity and diabetic foot infections.
• Community-acquired Pneumonia.
• Complicated Urinary Tract Infections including pyelonephritis.
• Acute Pelvic Infections including postpartum endomyometritis, septic abortion and post surgical gynecologic infections.
• Bacterial Septicaemia Prevention: Ertapenem is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery.
Dosage & Administration
Adults (>12 years): 1 gm given once a day.
Pediatric (3 months to 12 years): 15 mg/kg twice daily (not to exceed 1 g/day).
Ertapenem may be administered by IV infusion or IM injection. When administered intravenously, it should be infused over a period of 30 minutes.
IM administration of Ertapenem may be used as an alternative to IV administration in the treatment of those infections for which intramuscular therapy is appropriate.
The usual duration of therapy with Ertapenem is 3 to 14 days but varies by the type of infection and causative pathogen(s). When clinically indicated, a switch to an appropriate oral antimicrobial may be implemented if clinical improvement has been observed.
Prophylaxis of surgical site infection following elective colorectal surgery:
To prevent surgical site infections following surgery in adults, the recommended dosage is 1 g IV administered as a single intravenous dose given 1 hour prior to the surgical incision.
Patients with renal insufficiency: Ertapenem may be used for the treatment of infections in adult patients with renal insufficiency. In patients whose creatinine clearance is >30 mL/min no dosage adjustment is necessary. Adult patients with advanced renal insufficiency (creatinine clearance ≤30 mL/min), including those on haemodialysis, should receive 500 mg daily.
There are no data in paediatric patients with renal insufficiency.
Patients on Haemodialysis: If Ertapenem is given at least 6 hours prior to haemodialysis, no supplementary dose is needed. When adult patients on haemodialysis are given Ertapenem within 6 hours prior to haemodialysis, a supplementary 30% dose is recommended following the haemodialysis session. There are no data in patients undergoing peritoneal dialysis or haemofiltration. There are no data in paediatric patients on haemodialysis. When only the serum creatinine is available, the following formula may be used to estimate creatinine clearance.
Males: weight in kg x (140-age in years) / 72 x serum creatinine (mg/100 mL).
Females: 0.85Xvalue calculated for males.
No dosage adjustment is recommended in patients with impaired hepatic function.
Side Effects
Most adverse experiences reported in these clinical studies were described as mild to moderate in severity. The most common drug-related adverse experiences reported during parenteral therapy with Ertapenem include Headache, Infused vein complication, phlebitis/ thrombophlebitis, Diarrhoea, nausea and vomiting.
Other uncommon side effects include- Dizziness, somnolence, insomnia, seizure, confusion, Extravasation, hypotension, Dyspnoea, Oral candidiasis, constipation, acid regurgitation, C.
difficile-associated diarrhoea, dry mouth, dyspepsia, anorexia, Erythema, pruritus, Abdominal pain, taste perversion, asthenia/fatigue, candidiasis, oedema/swelling, fever, pain, chest pain, Vaginal pruritus.
Precautions
Before initiating therapy with Ertapenem, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, other beta-lactams and other allergens. If an allergic reaction to Ertapenem occurs, discontinue the drug immediately. Serious anaphylactic reactions require immediate emergency treatment. As with other antibiotics, prolonged use of Ertapenem may result in overgrowth of non-susceptible organisms. Repeated evaluation of the patient’s condition is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including Ertapenem, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea subsequent to the administration of antibacterial agents. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of “antibiotic-associated colitis”.
Use in Pregnancy & Lactation
Pregnancy: Ertapenem has been assigned to pregnancy category B by the FDA. There are no adequate and well-controlled studies in pregnant women. Ertapenem should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and fetus.
Lactation: Ertapenem is excreted in human milk. Caution should be exercised when Ertapenem is administered to a nursing woman.
Drug Interaction
When Ertapenem is administered with probenecid, probenecid competes for active tubular secretion and thus inhibits the renal excretion of Ertapenem. This leads to small but statistically significant increases in the elimination half-life (19%) and in the extent of systemic exposure (25%).
No dosage adjustment is necessary when Ertapenem is given with probenecid. Because of the small effect on half-life, the co-administration with probenecid to extend the half-life of Ertapenem is not recommended. Other than with probenecid, no specific clinical drug interaction studies have been conducted. Decreased serum levels of valproic acid with co-administration of Ertapenem have been reported. Careful monitoring of serum levels of valproic acid should be considered if Ertapenem is to be co-administered with valproic acid.
Over Dose
No specific information is available on the treatment of overdosage with Ertapenem. Intravenous administration of Ertapenem at a 3 g daily dose for 8 days to healthy adult volunteers did not result in significant toxicity. In pediatric clinical studies, a single IV dose of 40 mg/kg up to a maximum of 2 g did not result in toxicity.
In the event of an overdose, Ertapenem should be discontinued and general supportive treatment given until renal elimination takes place.
Ertapenem can be removed by haemodialysis; however, no information is available on the use of haemodialysis to treat overdosage.
Storage
For wandara injection: Store at 2-8 â° C. Keep away from light and out of the reach of children. For auxiliaries pack: Do not store above 30 â°C. Keep away from light and out of the reach of children.
Commercial Pack
Wandara TM : Each box contains one vial containing Ertapenem 1 gm. The auxiliaries box contains one vial of 60 ml normal saline, one sterile disposable syringe (10 ml), one infusion set with butterfly needle, a hanger, first aid band and alcohol pad.