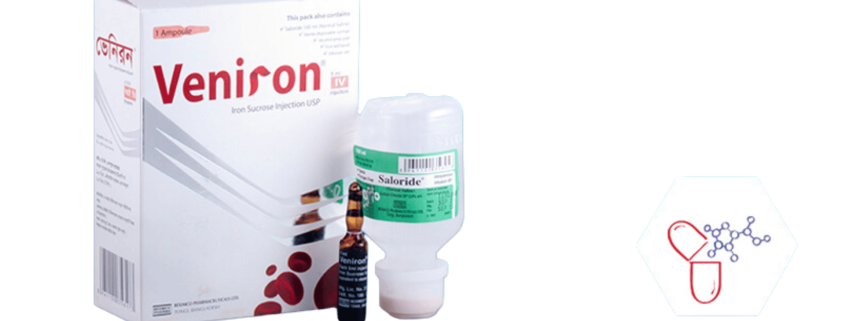
Veniron
Generic Name: Iron-sucrose 100 mg/5ml
Dosage Form: Injection
TG Name: Vitamins & Minerals Supplement
1. What Veniron® is and what it is used for?
Veniron® is a brown, sterile, aqueous, complex of Polynuclear Iron (III) Hydroxide in Sucrose for Intravenous use. The drug product contains approximately 30% Sucrose w/v (300 mg/ml).
Veniron® is indicated for the treatment of Iron deficiency in the following indications:
- Where there is a clinical need for a rapid Iron supply In patients who can not tolerate oral Iron therapy or who are non-compliant In active inflammatory bowel disease where oral Iron preparations are ineffective.
- Non-dialysis dependent-chronic kidney disease (NDD-CKD) patients receiving an erythropoietin
- Non-dialysis dependent-chronic kidney disease (NDD-CKD) patients not receiving an erythropoietin Hemodialysis dependent-chronic kidney disease (HDD-CKD) patients receiving an erythropoietin Peritoneal dialysis dependent-chronic kidney disease (PDD-CKD) patients receiving an erythropoietin.
- It is also indicated in the treatment of Iron deficiency anaemia in patients undergoing surgical procedures, patients donating blood, postpartum patients.
2. Before you take Veniron®
The use of Iron Sucrose is contraindicated in patients with evidence of Iron overload, in patients with known hypersensitivity to Iron Sucrose or any of its inactive components, and in patients with anaemia not caused by Iron deficiency. It is also contraindicated in patients with history of allergic disorders including asthma, eczema and anaphylaxis, liver disease and infections.
Take special care with Veniron®
Check with your doctor before taking this medicine if:
General: Because body Iron excretion is limited and excess tissue Iron can be hazardous, caution should be exercised to withhold Iron administration in the presence of evidence of tissue Iron overload. Patients receiving Iron Sucrose require periodic monitoring of hematologic and haematinic parameters (hemoglobin, hematocrit, serum ferritin and transferrin saturation). Iron therapy should be withheld in patients with evidence of Iron overload. Transferrin saturation values increase rapidly after IV administration of Iron Sucrose; thus, serum Iron values may be reliably obtained 48 hours after IV dosing. Hypersensitivity Reactions: Serious hypersensitivity reactions have been rarely reported in patients receiving Iron Sucrose. Several cases of mild or moderate hypersensitivity reactions were observed in these studies.
- Hypotension: Hypotension has been reported frequently in hemodialysis patients receiving intravenous Iron. Hypotension following administration of Iron Sucrose may be related to rate of administration and total dose administered. Caution should be taken to administer Iron Sucrose according to recommended guidelines.
- Pediatric Use: Safety and effectiveness of Iron Sucrose in pediatric patients have not been established.
- Geriatric Use: No overall differences in safety were observed between the elder subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Taking other medicines
Drug-drug interactions involving Iron Sucrose have not been studied. Iron Sucrose Injection should not be administered concomitantly with oral iron preparations since the absorption of oral Iron is reduced. Even oral Iron therapy should not be given until 5 days after last injection.
Pregnancy and breast-feeding
- Use in Pregnancy: Pregnancy Category-B. No adequate and well controlled studies in pregnant women. This drug should be used during pregnancy only if clearly needed.
- Use in Lactation: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Iron Sucrose is administered to a nursing woman.
3. How to take Veniron®?
Veniron® is exclusively to be administered intravenously by drip infusion, by slow injection or directly into the venous limb of the dialyser and is not suitable for intramuscular use and for total dose infusion (TDI), where the full dose of Iron required, representing the patient’s total Iron deficit is administered in one complete infusion. Before administration of the first therapeutic dose, a test dose should be given.
Dosage and administration
Normal Dosage
Adults and Elderly: 5-10 ml Veniron® (100-200 mg Iron) once to three times a week depending on the hemoglobin level.
Children: There is limited data on children under study conditions. If there is a clinical need, it is recommended not to exceed 0.15 ml Veniron® (3 mg Iron) per kg body weight once to three times per week depending on the haemoglobin level.
4. Possible side effects
Adverse reactions, whether or not related to Iron Sucrose injection are as follows: hypotension, cramps/leg cramps, nausea, -headache, vomiting, and diarrhea. Some of these symptoms may be seen in patients with chronic renal failure or on hemodialysis not receiving intravenous iron. Body as a Whole: headache, fever,
pain, asthenia, unwell, malaise, accidental injury. Cardiovascular Disorders, General: hypotension, chest pain, hypertension, hypervolemia. Gastrointestinal Disorders: nausea, vomiting, abdominal pain, elevated liver enzymes. Central and Peripheral Nervous System: dizziness. Musculoskeletal System: cramps/leg cramps, musculoskeletal pain. Respiratory System: dyspnea pneumonia, cough. Skin and appendages: pruritus, application site reaction. Hypersensitivity reactions: In safety studies, several patients experienced mild or moderate hypersensitivity reactions presenting with wheezing, dyspnea, hypotension, rashes, or pruritus. Anaphylactoid reactions including patients who experienced serious or life-threatening reactions (anaphylactic shock, loss of consciousness or collapse, bronchospasm with dyspnea, or convulsion) associated with Iron Sucrose administration can occur. So, patients should be given a small test dose initially.
5. How to store Veniron®?
Store in a cool (15° C- 30° C) & dry place, protected from light. Keep out of the reach of children. Do not freeze.