Tinobac(Spectinomycin)
Therapeutic Group: Anti Bacterial
Presentation
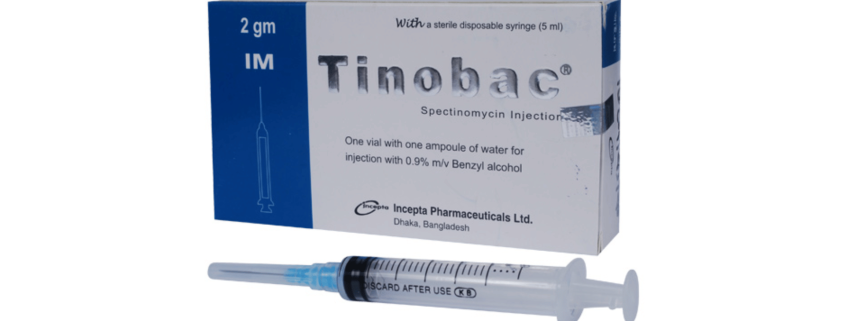
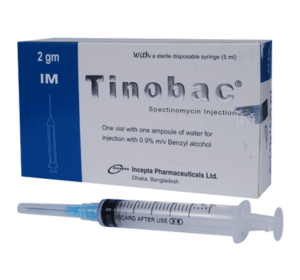
Tinobac 2 gm IM Injection: Each vial contains sterile powder of Spectinomycin Hydrochloride USP equivalent to Spectinomycin 2 gm.
Description
Tinobac Sterile Powder contains Spectinomycin Hydrochloride which is an aminocyclitol antibiotic produced by a species of soil microorganism designated as Streptomyces spectabilis.
Microbiology
Spectinomycin Hydrochloride is an inhibitor of protein synthesis in the bacterial cell; the site of action is the 30S ribosomal subunit. In vitro studies have shown Spectinomycin Hydrochloride to be active against most strains of Neisseria gonorrhoeae
Indications
Tinobac Sterile Powder is indicated in the treatment of:
Acute gonorrheal urethritis and proctitis in the male
Acute gonorrheal cervicitis and proctitis in the female
Men and women with known recent exposure to gonorrhea should be treated as those known to have gonorrhea.
Dosage & Administration
Reconstitution
Tinobac Sterile Powder, 2 grams: reconstitute with 3.2 ml of the accompanying water for injection with 0.9% Benzyl alcohol. Shake vials vigorously immediately after adding diluent and before withdrawing dose.
Dosage
Intramuscular injections should be made deep into the upper outer quadrant of the gluteal muscle.
Adults (Men and Women)-Inject 5 ml intramuscularly for a 2 gm dose.This is also the recommended dose for patients being treated after failure of previous antibiotic therapy
Side Effects
The following reactions were observed during the single dose clinical trials: soreness at the injection site, urticaria, dizziness, nausea, chills, fever and insomnia
Precautions
The usual precautions should be observed with atopic individuals. Prescribing Spectinomycin Sterile Powder in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria
Use in Pregnancy & Lactation
Pregnancy Category B. Since there are no controlled studies of Spectinomycin in pregnant women, and because animal reproduction studies are not always predictive of human responses, Spectinomycin should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Spectinomycin is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established.
Over Dose
Information on overdosage in humans is not available. Hemodialysis has been reported to aid in the removal of intravenously administered Spectinomycin from the body.
Commercial Pack
Tinobac 2 gm IM Injection: Each vial contains sterile powder of Spectionmycin Hydrochloride USP equivalent to Spectinomycin 2 gm accompanied with an ampoule of 3.2 ml Water For Injection with Benzyl alcohol 0.9% m/v and a 5 ml sterile disposable syringe.