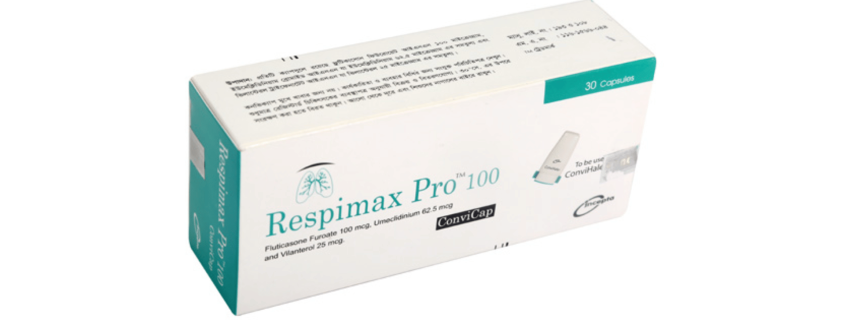
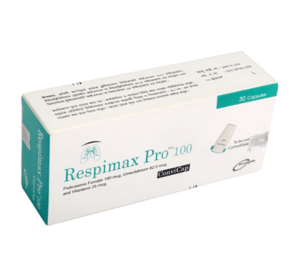
Respimax Pro ConviCap(Fluticasone Furoate, Umeclidinium and Vilanterol)
Therapeutic Group: Long acting Selective Beta-2 Adrenoceptor Stimulants
Presentation
Respimax ProTM 100 ConviCap: Each capsule contains Fluticasone Furoate INN 100 mcg, Umeclidinium Bromide INN equivalent to Umeclidinium 62.5 mcg and Vilanterol Trifenatate INN equivalent to Vilanterol 25 mcg.
Respimax ProTM 200 ConviCap: Each capsule contains Fluticasone Furoate INN 200 mcg, Umeclidinium Bromide INN equivalent to Umeclidinium 62.5 mcg and Vilanterol Trifenatate INN equivalent to Vilanterol 25 mcg.
Description
This is an inhalation powder drug product for delivery of a combination of fluticasone furoate, umeclidinium and vilanterol combination to patients by oral inhalation.
Fluticasone Furoate, a synthetic trifluorinated corticosteroid. Fluticasone Furoate is a white powder with a molecular weight of 538.6. It is practically insoluble in water. Umeclidinium Bromide is a white powder with a molecular weight of 508.5 (as a quaternary ammonium bromide compound). It is slightly soluble in water. Vilanterol Trifenatate is practically insoluble in water.
Indications
It is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease. It is indicated for the maintenance treatment of asthma in patients aged 18 years and older.
Dosage & Administration
COPD: The maximum recommended dose is Respimax ProTM 100 ConviCap one capsule to be inhaled once daily.
Asthma: The maximum recommended dose is Respimax Pro 100 ConviCap or Respimax Pro 200 ConviCap one capsule to be inhaled once daily.
Side Effects
COPD: Most common adverse reactions (incidence ≥1%) are upper respiratory tract infection, pneumonia, bronchitis, oral candidiasis, headache, back pain, arthralgia, influenza, sinusitis, pharyngitis, rhinitis, dysgeusia, constipation, urinary tract infection, diarrhea, gastroenteritis, oropharyngeal pain, cough, and dysphonia.
Asthma: Most common adverse reactions (incidence ≥2%) are pharyngitis/nasopharyngitis, upper respiratory tract infection/viral upper respiratory tract infection, bronchitis, respiratory tract infection/viral respiratory tract infection, sinusitis/acute sinusitis, urinary tract infection, rhinitis, influenza, headache, and back pain.
Precautions
Do not use to treat acute symptoms. Do not use in combination with additional therapy containing a LABA because of risk of overdose. Increased risk of pneumonia in patients with COPD. Monitor patients for signs and symptoms of pneumonia.
Use in Pregnancy & Lactation
Pregnancy: This medicinal product should only be used during pregnancy if the expected benefit to the patient justifies the potential risk to the foetus.
Lactation: A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from therapy, taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Drug Interaction
No specific interaction studies were conducted with indacaterol/glycopyrronium/mometasone furoate. Information on the potential for interactions is based on the potential for each of the monotherapy components.
Over Dose
Treatment of overdosage consists of discontinuation of therapy together with institution of appropriate symptomatic and/or supportive therapy.
Storage
Do not store above 30 °C.
Keep away from light and out of the reach of children.
Protect from freezing.
Insert the ConviCap in the ConviHaler just prior to use to protect from deterioration by moisture.
Commercial Pack
Respimax ProTM 100 ConviCap: Each box contains 5 Alu-Alu blister strips of 6 capsules. Respimax ProTM 200 ConviCap: Each box contains 5 Alu-Alu blister strips of 6 capsules.