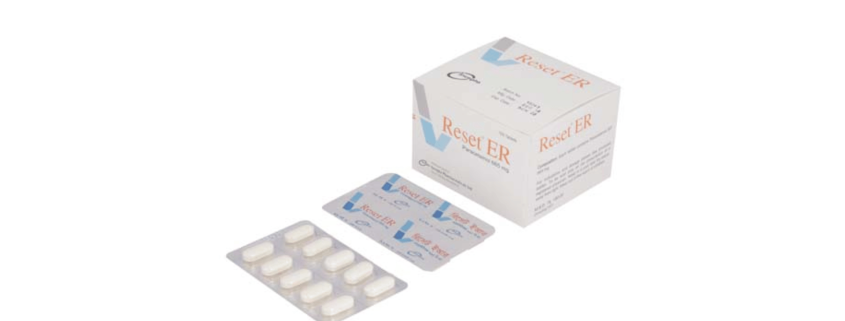
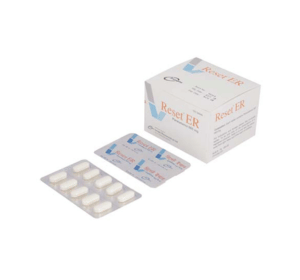
Reset ER(Paracetamol)
Therapeutic Group: Analgesic, Anti Inflammatory
Presentation
Reset ER: Each tablet contains Paracetamol BP 665mg.
Description
Reset ER is the preparation of Paracetamol 665 mg extended release formulation of bilayer tablet. Immediate release formulation for immediate response and extended release formulation for extended response of 8 hours.
Indications
Reset ER is effective for the relief of persistent pain associated with osteoarthritis and muscle aches and pains such as backache. It also provides effective temporary relief from the pain and discomfort associated with headache, tension headache, period pain, toothache and pain after dental procedures, cold & flu. Reset ER tablet is also effective in reducing fever.
Dosage & Administration
Adults and children over 12 years: 2 tablets, swallowed whole, every 6 to 8 hours (maximum of 6 tablets in any 24 hours). The tablets must not be crushed.
Precautions
Reset ER should be administered with caution to patients with hepatic or renal dysfunction.
Use in Pregnancy & Lactation
Reset ER tablet has been categorized as Category A.Lactation: Paracetamol is excreted in breast milk. It has been reported that from a single 665 mg dose, 0.04 to 0.23% will be available for ingestion by the infant. These results are based on immediate release preparations of paracetamol. There is no data available on the excretion of extended release paracetamol preparations in breast milk.
Drug Interaction
Anticoagulant dosage may require reduction if paracetamol medication is prolonged. Paracetamol absorption from immediate release preparations is increased by drugs which increase gastric emptying e.g. metoclopramide, and decreased by drugs which decrease gastric emptying, e.g. propantheline, antidepressants with anticholinergic properties, narcotic analgesics. However, concurrent administration of metoclopramide may reduce the absorption of paracetamol from this extended release dosage form, as it accelerates gastric emptying and intestinal transit. Paracetamol may increase chloramphenicol concentrations. The likelihood of paracetamol toxicity may be increased by the concomitant use of enzyme inducing agents such as alcohol or anticonvulsant drugs.
Over Dose
Paracetamol overdose may cause hepatic failure due to prolonged exposure of paracetamol in the blood from the extended release form.Immediate medical management is required in the event of overdose even symptoms of overdose are not present.
Storage
Do not store above 30 o C. Keep away from light and out of the reach of children.
Commercial Pack
Reset ER: Each box contains 10 alu-pvc blister strips of 10 tablets
Others
Reports of adverse reactions are rare. Although the following adverse reactions have been reported, a causal relationship to the administration of paracetamol has been neither confirmed nor refuted: dyspepsia, nausea, allergic and haematological reactions.