Remdinil™
Remdesivir
Influenza Antiviral (Systemic Antiviral)
Indication:
For the treatment of suspected or laboratory confirmed Corona Virus Disease 2019 (COVID-19) in adult and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation (SpO2) ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). Specifically, Remdesivir is only authorized for hospitalized adults and pediatric patients for whom use of an intravenous agent is clinically appropriate.
Dosage & Administration:
The recommended dosage in adults is a single loading dose of Remdesivir 200 mg (two vials of Remdinil™ 100 mg solution for IV infusion) on Day 1 followed by once-daily maintenance doses of Remdesivir 100 mg (one vial of Remdinil™ 100 mg solution for IV infusion) from Day 2 via IV infusion. For patients requiring invasive mechanical ventilation and/or ECMO, total treatment duration is 10 days. For patients not requiring invasive mechanical ventilation and/or ECMO, total treatment duration is 5 days. If a patient does not demonstrate clinical improvement, treatment may be extended for up to 5 additional days (i.e., up to a total of 10 days). Administer Remdesivir via IV infusion in a total volume of up to 250 mL 0.9% sodium chloride over 30 to 120 minutes.
Do not use Remdinil™ 100 mg solution for IV infusion for pediatric patients weighing 3.5 kg to less than 40 kg.
Preparation:
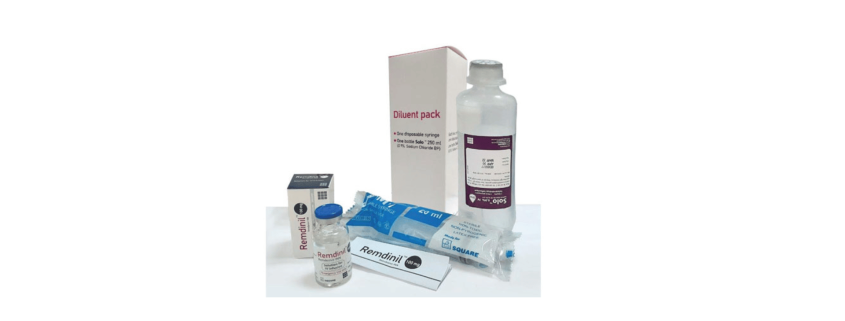
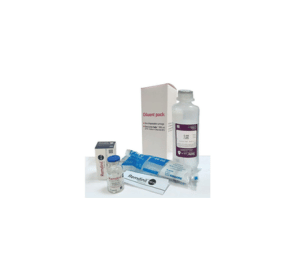
Remdinil™ 100 mg solution for IV infusion: 1 vial filled with Remdesivir 100 mg concentrate for solution for IV infusion, 20 ml disposable syringe & 250 ml 0.9% Sodium Chloride (Solo™) infusion bottle.