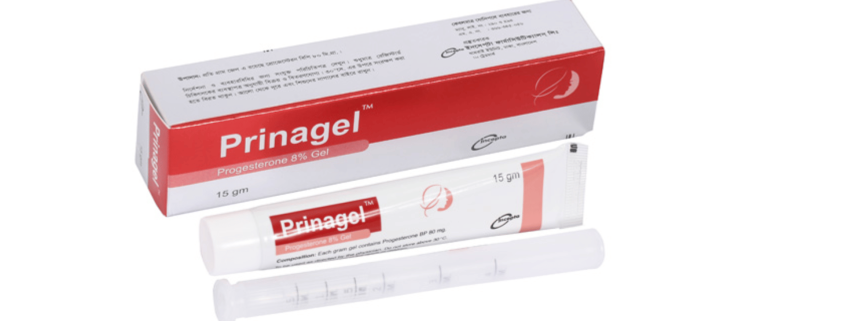
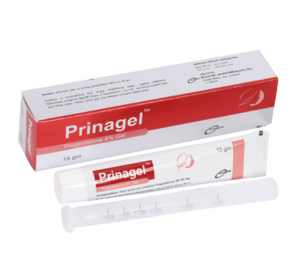
Prinagel(Progesterone 8% Gel)
Therapeutic Group: Gynaecological
Presentation
Prinagel : Each gram gel contains Progesterone BP 80 mg.
Description
Prinagel is a micronized progesterone in an emulsion system, which is used through polypropylene vaginal applicators. Progesterone is a naturally occurring steroid that is secreted by the ovary, placenta, and adrenal gland and essential for the development of decidual tissue. Progesterone acts to maintain the pregnancy, decreases the circulatory levels of gonadotropins.
Indications
• Prinagel 8% is indicated for progesterone supplementation or replacement as part of an Assisted Reproductive Technology (“ART”) treatment for infertile women with progesterone deficiency.
• Prinagel 8% is also indicated for the treatment of secondary amenorrhea.
Dosage & Administration
Assisted Reproductive Technology
Prinagel 8% is administered vaginally at a dose of 90 mg progesterone once daily in women who require progesterone supplementation. Prinagel 8% is administered vaginally at a dose of 90 mg progesterone twice daily in women with partial or complete ovarian failure who require progesterone replacement. If pregnancy occurs, treatment may be continued until placental autonomy is achieved, up to 10-12 weeks.
Secondary Amenorrhea
Prinagel 8% is administered vaginally every other day up to a total of six doses.
Side Effects
Common side effects include abdominal pain, perineal pain (the perineum is the area between the vagina and the rectum), cramps, bloating, headache, fatigue, increased appetite, constipation, diarrhea, nausea, joint pain, depression, mood swings, sleep disorder, nervousness, decreased libido, breast enlargement, excessive urination at night, vaginal discharge, upper respiratory tract infection.
Precautions
The pretreatment physical examination should include special reference to breast and pelvic organs, as well as Papanicolaou smear. In cases of breakthrough bleeding, as in all cases of irregular vaginal bleeding, nonfunctional causes should be considered. In cases of undiagnosed vaginal bleeding, adequate diagnostic measures should be undertaken. Because progestogens may cause some degree of fluid retention, conditions which might be influenced by this factor (e.g., epilepsy, migraine, asthma, cardiac or renal dysfunction) require careful observation. The pathologist should be advised of progesterone therapy when relevant specimens are submitted. Patients who have a history of psychic depression should be carefully observed and the drug discontinued if the depression recurs to a serious degree. A decrease in glucose tolerance has been observed in a small percentage of patients on estrogen progestin combination drugs. The mechanism of this decrease is not known. For this reason, diabetic patients should be carefully observed while receiving progestin therapy.
Use in Pregnancy & Lactation
If pregnancy occurs, Prinagel TM may be continued until placental autonomy is achieved, up to 10-12 weeks. Detectable amounts of progestins have been identified in the milk of mothers receiving them. The effect of this on the nursing infant has not been determined.
Drug Interaction
No drug interactions have been assessed with PrinagelTM
.
Over Dose
There have been no reports of overdosage with PrinagelTM. In the case of overdosage, however, discontinue Prinagel TM, treat the patient symptomatically, and institute supportive measures. As with all prescription drugs, this medicine should be kept out of the reach of children.
Storage
Do not store above 30°C. Keep away from light and out of the reach of children.
Commercial Pack
Prinagel : Each pack contains 15 gm of Progesterone 8% vaginal gel and a vaginal applicator.
Others
Step 1: Wash hand thoroughly with soapy water
Step 2: Open the sealed wrapper and remove the applicator
Step 3: Insert the tip of the applicator into the tube
Step 4: Press down the tube and squeeze the specified amount of gel (1.5 gm indicated marking) into the applicator tube
Step 5: Remove the applicator from the tube
Step 6: Gently insert the rounded tip of the applicator into vagina
Step 7: Push the plunger to release the gel
Step 8: After releasing the gel remove the applicator, keep the applicator clean and germfree for later use.