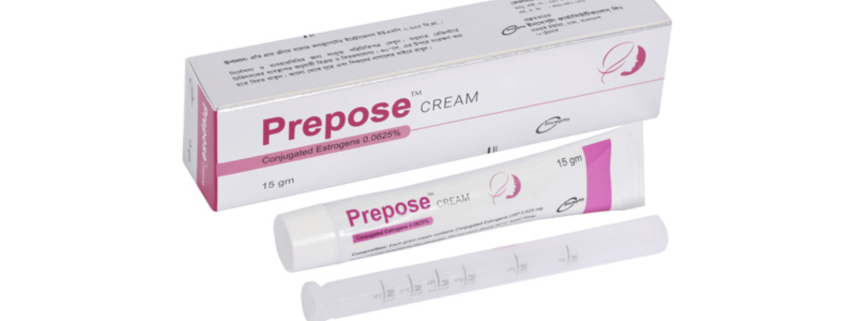
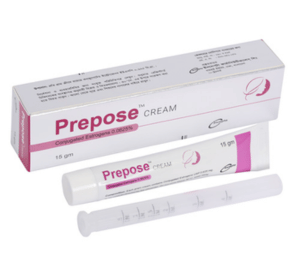
Prepose CREAM(Conjugated Estrogens 0.0625%)
Therapeutic Group: Gynaecological
Presentation
Prepose cream: Each gram cream contains conjugated Estrogens USP 0.625 mg.
Description
Conjugated Estrogens is a mixture of estrogens obtained exclusively from natural sources, blended to represent the average composition of material derived from pregnant mares’ urine. It contains the sodium salts of water-soluble sulfate esters of estrone, equilin, and 17-alpha dihydroequilin, together with smaller amounts of 17-alpha estradiol, equilenin, 17-alpha dihydroequilenin, 17-beta dihydroequilin, 17-beta dihydroequilenin, 17-beta estradiol, and delta 8, 9-dehydroestrone.
Indications
PreposeTM (Conjugated Estrogens 0.625 mg, vaginal cream) is indicated for-
• Treatment of Atrophic Vaginitis and Kraurosis Vulvae
• Treatment of Moderate to Severe Dyspareunia, a symptom of Vulvar and Vaginal Atrophy, due to Menopause.
Dosage & Administration
• Treatment of Atrophic Vaginitis and Kraurosis Vulvae:
– Cyclic administration of 0.5 to 2 g intravaginally [daily for 21 days, then off for 7 days]
• Treatment of Moderate to Severe Dyspareunia, a Symptom of Vulvar and Vaginal Atrophy Due to Menopause:
– Cyclic administration of 0.5 g intravaginally [daily for 21 days, then off for 7
days]
– Twice-weekly administration of 0.5 g intravaginally [for example, Monday and
Thursday]
Side Effects
The most common adverse reactions are headache, pelvic pain, vasodilation, breast pain, leucorrhea, metrorrhagia, vaginitis, and vulvovaginal disorder.
Precautions
• Estrogens increase the risk of gallbladder disease
• Discontinue estrogen if severe hypercalcemia, loss of vision, severe hypertriglyceridemia or cholestatic jaundice occurs.
• Monitor thyroid function in women on thyroid replacement therapy.
Use in Pregnancy & Lactation
Prepose is contraindicated in pregnancy. As a general principle, estrogen administration to nursing women has been shown to decrease the quantity and quality of breast milk.
Drug Interaction
Rifampin reportedly decreases estrogenic activity during concomitant use with estrogen. This effect has been attributed to enhanced metabolism of estrogen, presumably by induction of hepatic microsomal enzymes.
Over Dose
Overdosage with estrogen may cause nausea, breast discomfort, fluid retention, bloating, or vaginal bleeding in women. Treatment of overdose consists of discontinuation of PreposeTM therapy together with institution of appropriate symptomatic care.
Storage
Do not store above 30°C. Keep away from light and out of the reach of children.
Commercial Pack
Prepose cream: Each pack contains 15 gm of Conjugated Estrogens 0.0625% vaginal cream and a vaginal applicator.
Others
Instructions for Applicator Use:
Step 1: Wash your hands thoroughly with soapy water
Step 2: Open the sealed wrapper and remove the applicator
Step 3: Insert the tip of the applicator into the tube
Step 4: Press down the tube and squeeze the specified amount of gel (0.5 gm indicated marking) into the applicator tube.
Step 5: Remove the applicator from the tube
Step 6: Gently insert the rounded tip of the applicator into vagina
Step 7: Push the plunger to release the gel
Step 8: After releasing the gel, remove the applicator. Keep the applicator clean and germ-free for later use.