Pralidox(Pralidoxime Chloride)
Therapeutic Group: Antidote Preparation
Presentation
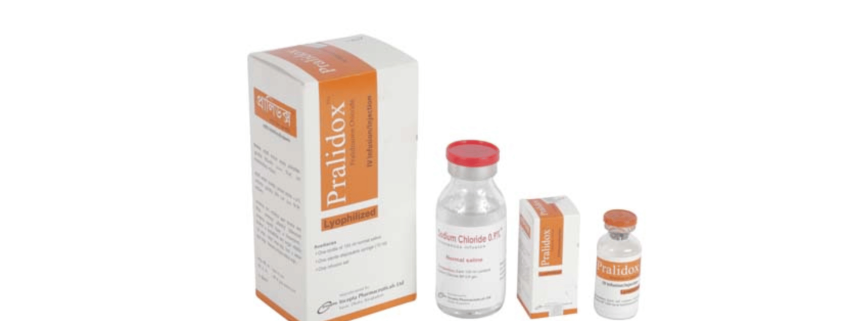
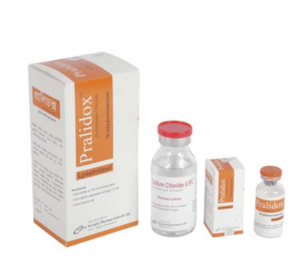
Pralidox TM IV Infusion/Injection: Each vial contains Pralidoxime Chloride USP 1000 mg as lyophilized powder.
Description
Pralidoxime Chloride is an acetylcholinesterase reactivator. The principal action of Pralidoxime Chloride is to reactivate acetylcholinesterase (mainly outside of the central nervous system) which has been inactivated by phosphorylation due to an organophosphate pesticide or related compound. The destruction of accumulated acetylcholine can then proceed, and neuromuscular junctions will again function normally. Pralidoxime Chloride also slows the process of “aging†of phosphorylated cholinesterase to a nonreactivatable form, and detoxifies certain organophosphates by direct chemical reaction. The drug has its most certain effect in relieving paralysis of the muscles of respiration. Atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site.
Pharmacokinetic properties: Minimum therapeutic concentration of Pralidoxime Chloride in plasma (4 µg/ml) may be reached in about 16 minutes after injection of 600 mg Pralidoxime Chloride. The drug is rapidly excreted in urine partly unchanged and partly as metabolite produced by the liver.
Indications
Pralidox is indicated as an antidote:
(1) In the treatment of poisoning due to those pesticides and chemicals of the organophosphate class which have anticholinesterase activity.
(2) In the control of overdose by anticholinesterase drugs used in the treatment of myasthenia gravis.
Dosage & Administration
Adults: Inject an initial dose of 1000 to 2000 mg of Pralidox (Pralidoxime Chloride), preferably as an infusion in 100 ml of normal saline, over a 15 to 30 minute period. If this is not practical or pulmonary edema is present the dose should be given slowly (over not less than 5 minutes) by intravenous injection, as 50 mg/ml solution (e.g. 1000 mg in 20 ml). A second dose of 1000 to 2000 mg may be indicated after about one hour if muscle weakness has not been relieved. Additional doses may be given every 10-12 hours if muscle weakness persists.
Children 16 years and under : Loading dose: 20-50 mg/kg (maximum: 2000 mg/dose) over 15 -30 minutes.
Maintenance infusion: 10-20 mg/kg/hour; alternatively, a repeat bolus of 20-50 mg/kg (maximum 2000 mg/dose) may be administered after 1 hour and repeated every 10-12 hours thereafter, as needed. Children >16 years age: Refer to adult dosing.
Preparation of administration: For intravenous infusion: Reconstitute a single Pralidox (Pralidoxime Chloride) 1000 mg vial by adding 20 ml of Normal Saline (0.9% Sodium Chloride solution) for Injection through 10 ml syringe, which results in a 50 mg/ml concentration. The solution should be further diluted with remaining Normal Saline to achieve a concentration of 10 to 20 mg/ml (e.g. 1000 mg in 100 ml or 2000 mg in 100 ml). For fluid restricted patients or for rapid administration (over at least 5 min), a maximum concentration of 50 mg/ml may be used. Discard unused solution after dose has been withdrawn.
Side Effects
Certain side effects such as tachycardia, laryngospasm and muscle rigidity have been attributed in a few cases to a too-rapid rate of injection.
Precautions
The following precautions should be kept in mind in the treatment of acetylcholinesterase poisoning, although they do not bear directly on the use of Pralidoxime Chloride, since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsion; morphine, theophylline, aminophylline, succinylcholine, reserpine and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported with patients when succinylcholine is given with drugs having anticholinesterase activity; therefore it should be used with cautions.
Use in Pregnancy & Lactation
Pregnancy: Pregnancy category C. Pralidoxime Chloride should be given to a pregnant woman only if clearly needed.
Lactation: It is not known whether this drug is excreted in human milk. So, caution should be exercised when pralidoxime chloride is administered to a nursing woman.
Drug Interaction
When atropine and Pralidoxime Chloride are used together the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of Pralidoxime Chloride has been delayed.
Over Dose
Observed in normal subjects only: Dizziness, blurred vision, diplopia, headache, impaired accommodation, nausea, slight tachycardia. Treatment of overdose is artificial respiration and other supportive therapy should be administered as needed.
Storage
Store in a cool and dry place, away from light. Keep out of the reach of children.
Commercial Pack
PralidoxTM IV Infusion/Injection: Each box contains 1 vial of Pralidoxime Chloride 1000 mg, 1 bottle of 100ml normal saline (0.9% Sodium Chloride solution), 1 bottle hanger, a 10ml disposable syringe and 1 infusion set.