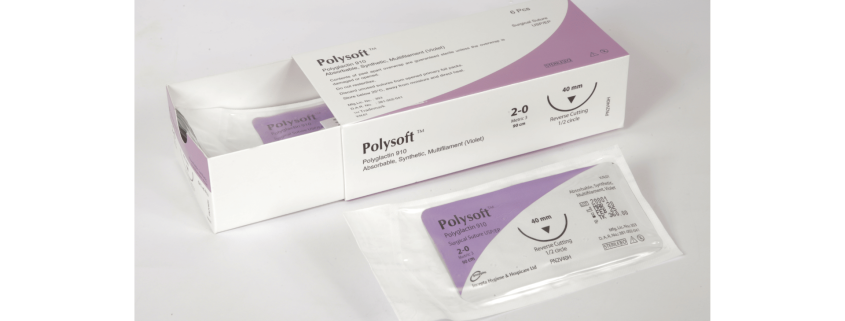
Polysoft 2-0(Absorbable Synthetic Multifilament, Polyglactin 910 (Violet))
Therapeutic Group: Suture
Presentation
Absorbable Synthetic Multifilament, Polyglactin 910 (Violet)
Description
Coated Polysoft (polyglactin 910) is a synthetic absorbable sterile surgical suture, composed of a copolymer made from 90% glycolide and 10% L-lactide. This suture is prepared by coating the suture material with a mixture composed of equal parts of copolymer of glycolide and lactide (polyglactin 370) and calcium stearate. Polyglactin 910 copolymer and polyglactin 370 with calcium stearate have been found to be nonantigenic, nonpyrogenic and elicit only a mild tissue reaction during absorption.
Indications
Coated Polysoft suture is indicated for use in general soft tissue approximation and/or ligation, including use in ophthalmic procedures, but not for use in cardiovascular and neurological tissues.
Dosage & Administration
N/A
Precautions
In handling this or any other suture material, care should be taken to avoid damage from handling. Avoid crushing or crimping damage due to application of surgical instruments such as forceps or needle holders. Coated Polysoft sutures which are treated to enhance handling characteristics, require the accepted surgical technique of flat and square ties with additional throws as warranted by surgical circumstance and the experience of the surgeon. Avoid prolonged exposure to elevated temperatures. To avoid damaging needle points and swage areas, grasp the needle in an area one-third (1/3) to one-half (1/2) of the distance from the swaged end to the point. Reshaping needles may cause them to lose strength and be less resistant to bending and breaking. Discard used needles in waste bin.
Storage
Store this suture below 250 C.
Keep away from moisture and direct heat.
Do not use if the date is expired.
Commercial Pack
Each box contains 6, 12 packs of Polyglactin 910 (Violet)
suture.
Others
WARNINGS :
Users should be familiar with surgical procedures and techniques involving absorbable sutures before employing coated Polysoft suture for wound closure, as risk of wound dehiscence may vary with the site of application and the suture material used. The use of this suture may be inappropriate in elderly, malnourished, or debilitated patients, or in patients suffering from conditions which may delay wound healing. As this is an absorbable suture material, the use of supplemental non-absorbable sutures should be considered by the surgeon in the closure of the sites which may undergo expansion, stretching or distention, or which may require additional support. Do not resterilize. Discard opened packages and unused sutures. As with any foreign body, prolonged contact of any suture with salt solutions, such as those found in the urinary or biliary tracts, may result in calculus formation. As an absorbable suture, coated Polysoft suture may act transiently as a foreign body. Acceptable surgical practice should be followed for the management of contaminated or infected wounds
ADVERSE REACTIONS:
Adverse effects associated with the use of this device include wound dehiscence, failure to provide adequate wound support in closure of the sites where expansion, stretching, or distension occur, failure to provide adequate wound support in elderly, malnourished or debilitated patients or in patients suffering from conditions which may delay wound healing, infection, minimal acute inflammatory tissue reaction, localized irritation when skin sutures are left in place for greater than 7 days, suture extrusion and delayed absorption in tissue with poor blood supply, calculi formation in urinary and biliary tracts when prolonged contact with salt solutions such as urine and bile occurs and transitory local irritation at the wound site.