Ivanor™
Ivabradine
Antianginal Drugs (Cardiovascular Preparations)
Indication:
Symptomatic treatment of chronic stable angina pectoris in coronary artery disease patients with normal sinus rhythm. Ivanor™ is indicated:
– In patients unable to tolerate or with a contra-indication to the use of beta-blockers, or
– In combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose and whose heart rate is > 60 bpm.
Dosage & Administration:
The usual recommended starting dose of Ivabradine is 5 mg twice daily which may be increased after 3-4 weeks of treatment to 7.5 mg twice daily, depending on therapeutic response. Usual dose is 1 tablet in the morning and 1 tablet in the evening during meals.
If the heart rate decreases persistently below 50 bpm at rest or if symptoms related to bradycardia, the dose must be adjusted downwards to 2.5mg twice daily (one half of the 5 mg tablet twice daily). Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist. Elderly: Consider a lower starting dose (2.5 mg twice daily i.e. one half 5 mg tablet twice daily).
Renal insufficiency: Use with caution in patients with creatinine clearance less than 15 ml/min.
Hepatic Impairment: Use with caution in patients with moderate hepatic impairment; contraindicated in severe hepatic impairment.
Children and adolescents: Not recommended.
Preparation:
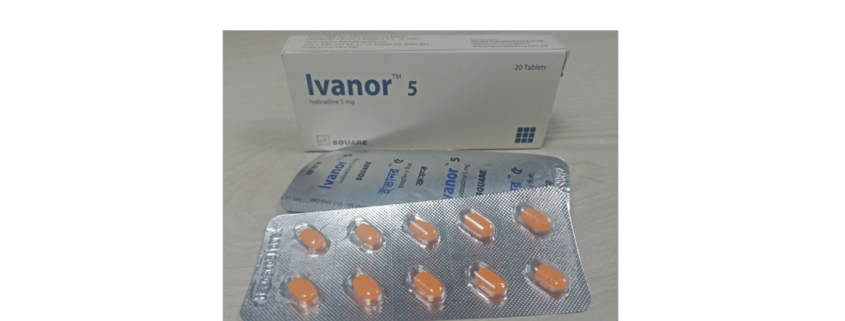
Ivanor ™ 5 tablet: Each box containing 20 film coated tablets in Alu-PVDC blister pack.
Ivanor ™ 7.5 tablet: Each box containing 10 film coated tablets in Alu-PVDC blister pack.