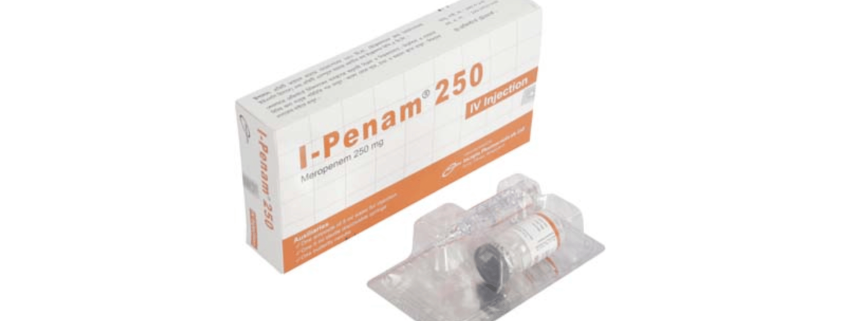
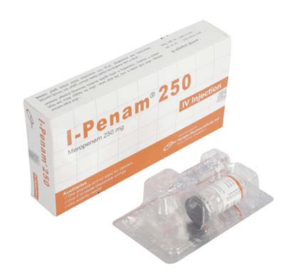
I-Penam(Meropenem)
Therapeutic Group: Anti Bacterial
Presentation
I-Penam 250 mg IV Injection: Each vial contains Meropenem 250 mg (as Meropenem for Injection USP).
I-Penam 500 mg IV Injection: Each vial contains Meropenem 500 mg (as Meropenem for Injection USP).
I-Penam 1 gm IV Injection: Each vial contains Meropenem 1 gm (as Meropenem for Injection USP).
Description
Meropenem is a carbapenem antibiotic for parenteral use, that is relatively stable to human dehydropeptidase-1 (DHP-1) and therefore does not require the addition of an inhibitor of DHP-1. Meropenem exerts its bactericidal action by interfering with vital bacterial cell wall synthesis. The ease with which it penetrates bacterial cell walls, its high level of stability to all serine beta-lactamases and its marked affinity for the Penicillin Binding Proteins (PBPs) explain the potent bactericidal action of meropenem against a broad spectrum of aerobic and anaerobic bacteria
Indications
Meropenem IV is indicated for treatment in adults and children for the following infections caused by single or multiple bacteria sensitive to meropenem.
– Pneumonia and Nosocomial Pneumonia
– Urinary Tract Infections
– Intra-abdominal Infections
– Gynaecological Infections, such as endometritis and pelvic inflammatory disease
– Skin and Skin Structure Infections
– Meningitis
Septicaemia
Empiric treatment, for presumed infections in adult patients with febrile neutropenia, used as monotherapy or in combination with anti-viral or anti-fungal agents.
Dosage & Administration
The dosage and duration of therapy shall be established depending on type and severity of infection and the condition of the patient. The recommended daily dosage is as follows:-
Adults: The adult dose is 500 mg to 1 gm by intravenous infusion over 15-30 minutes or as intravenous bolus (5 to 20 mL) over 3-5 minutes every 8 hours.
Pneumonia, urinary tract infections, gynaecological infections such as endometritis, skin and skin structure infections: 500 mg IV every 8 hours.
Nosocomial pneumonias, peritonitis, presumed infections in neutropenic patients and septicaemia: 1g IV every 8 hours.
Intra-abdominal infections: 500 mg to 1 gm every 8 hours.
Meningitis: 2 gm IV every 8 hours.
Renal impairment: No dosage adjustment is required for the elderly with normal renal function or creatinine clearance values above 50 ml/min. CrCl 26-50 mL/min, 1 gm every 12 hours; CrCl 10-25 mL/min, 500 mg every 12 hours; and CrCl <10 mL/min, 500 mg every 24 hours.
Hepatic impairment: No dosage adjustments are necessary with impairment of liver function.
Hemodialysis patients should receive meropenem after dialysis has been completed.
Children: Children 3 months to 12 years: 10 to 40 mg/kg intravenously every 8 hours depending on type and severity of infection, susceptibility of the pathogen(s) and the condition of the patient.
Intra-abdominal infections: 20 mg/kg every 8 hours.
Cystic fibrosis (4-18 years): 25-40 mg/kg every 8 hours.
Meningitis: 40 mg/kg IV every 8 hours.
Children over 50 kg weight: use adult dosage.
There is no experience in children with hepatic or renal impairment.
Elderly: No dosage adjustments are necessary in elderly patients unless creatinine clearance is <51 mL/min.
Side Effects
Meropenem is generally well tolerated. Local injection site reactions, rash, pruritus, urticaria, abdominal pain, nausea, vomiting, diarrhoea, pseudomembranous colitis. Rarely erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. Headache, paraesthesia and infrequently convulsions (although no causal relationship has been established). Oral and vaginal candidosis. Reversible thrombocythaemia, leucopenia, eosinophilia, thrombocytopenia and neutropenia (including rare cases of agranulocytosis). Positive Coombs test. Reduction in partial thromboplastin.
Rarely systemic allergic reactions (hypersensitivity), which may include angioedema and manifestations of anaphylaxis.
Precautions
If an allergic reaction to meropenem occurs, the drug should be discontinued and appropriate measures taken. Use of Meropenem in patients with hepatic disease should be made with careful monitoring of transaminase and bilirubin levels.
Use in Pregnancy & Lactation
Pregnancy: The safety of Meropenem in human pregnancy has not been evaluated. Meropenem should not be used in pregnancy unless the potential benefit justifies the potential risk to the foetus. In every case, it should be used under the direct supervision of the physician.
Lactation: Meropenem is detectable at very low concentrations in animal breast milk. Meropenem should not be used in breast-feeding women unless the potential benefit justifies the potential risk to the baby.
Drug Interaction
Probenecid competes with meropenem for active tubular secretion and thus inhibits the renal excretion, with the effect of increasing the elimination half-life and plasma concentration of meropenem. Meropenem may reduce serum valproic acid levels. Sub therapeutic levels may be reached in some patients.
Over Dose
Accidental overdosage could occur during therapy, particularly in patients with renal impairment. Treatment of overdosage should be symptomatic. In normal individuals, rapid renal elimination will occur; in subjects with renal impairment, haemodialysis will remove meropenem and its metabolite.
Commercial Pack
I-Penam 250 mg IV Injection: Each pack contains 1 vial of Meropenem for Injection USP equivalent to Meropenem 250 mg, 1 ampoule of 5 ml Water for Injection BP, a sterile disposable syringe (5 ml) and a butterfly needle.
I-Penam 500 mg IV Injection: Each pack contains 1 vial of Meropenem for Injection USP equivalent to Meropenem 500 mg, 1 ampoule of 10 ml Water for Injection BP, a sterile disposable syringe (10 ml) and a butterfly needle.
I-Penam 1 gm IV Injection: Each pack contains 1 vial of Meropenem for Injection USP equivalent to Meropenem 1 gm, 2 ampoules of 10 ml Water for Injection BP, a sterile disposable syringe (20 ml) and a butterfly needle.
Others
Method of Reconstruction:
For IV injection
I-Penam 500 mg should be reconstituted with 10 ml sterile water for injection. The reconstituted solutions are clear or pale yellow. It should be administered intravenously over 3-5 minutes.
I-Penam 1 gm should be reconstituted with 20 ml sterile water for injection. The reconstituted solutions are clear or pale yellow. It should be administered intravenously over 3-5 minutes.
For IV infusion
For intravenous infusion I-Penam may be reconstituted within the vial and then the resulting solution should be added to an I.V. container and further diluted to 50-200 ml with a compatible infusion fluid, as needed. It should be administered over 15-30 minutes.
Compatible infusion fluid
Meropenem IV is compatible with the following infusion fluids:
0.9% sodium chloride intravenous infusion
5% or 10% glucose intravenous infusion
5% glucose intravenous infusion with 0.02% sodium bicarbonate
5% glucose and 0.9% sodium chloride intravenous infusion
5% glucose with 0.225% sodium chloride intravenous infusion
5% glucose with 0.15% potassium chloride intravenous infusion
2.5% and 10% mannitol intravenous infusion
normosol-M in 5% glucose intravenous infusion.