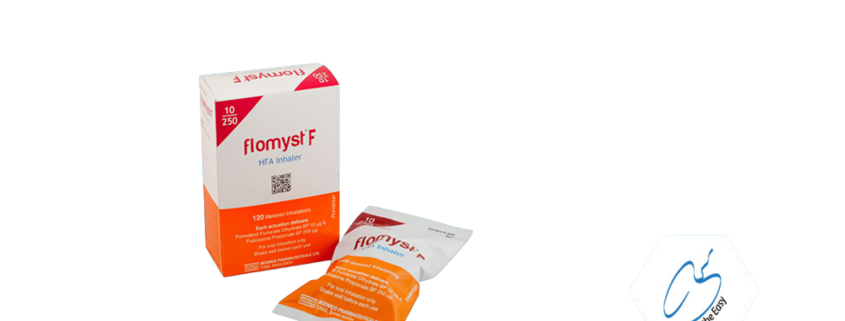
Flomyst F

Generic Name: Formoterol Fumarate Dihydrate and Fluticasone Propionate
Dosage Form: HFA Inhalation Aerosol
TG Name: Respiratory
What Flomyst® F Inhaler is and what it is used for ?
Flomyst® F is an inhaler (a pressurized inhalation, suspension) which contains two active ingredients Fluticasone propionate and Formoterol Fumarate Dihydrate. Fluticasone propionate which belongs to a group of medicines called steroids. Steroids help to reduce swelling and inflammation in the lungs. Formoterol Fumarate Dihydrate which belongs to a group of medicines called long-acting β2 agonists. Long-acting β2 agonists are long-acting bronchodilators which help the airways in your lungs to stay open, making it easier for you to breathe. Together these two active ingredients help to improve your breathing.Flomyst® F inhaler is indicated in the regular treatment of asthma
How to take Flomyst® F Inhaler ?
Flomyst® F 5/50 and 5 /125 HFA Inhaler are indicated in adults and adolescents aged 12 years and above. Flomyst® F 10/250 HFA Inhaler is indicated in adults only.
Recommended dose for adults and adolescents aged 12 years and above: Flomyst® F 5/50 & 5/125 HFA Inhaler – two inhalations (puffs) twice daily normally taken in the morning and in the evening.
For adults only: The total daily dose can be further increased if asthma still remains poorly controlled by administering the highest strength of this combination product – i.e. Flomyst® F 10/250 HFA Inhaler- two inhalations (puffs) twice daily. This highest strength is for use in adults only; it should not be used in adolescents aged 12 years and above.
Flomyst® F inhaler should not be used in children under 12 years.
Possible side effects
Potentially serious side-effects are hyperglycaemia; depression; aggression; paradoxical bronchospasm; agitation; vertigo; palpitations; ventricular extrasystoles; angina pectoris; tachycardia; hypertension; dyspnoea; peripheral oedema; Cushings syndrome; adrenal suppression; growth retardation; cataract and glaucoma; hypersensitivity reactions and QTc interval prolongation.
Pregnancy and Lactation
Administration of this inhaler is not recommended during pregnancy, and should only be considered if expected benefit to the mother is greater than any possible risk to the fetus. If this is the case, then the lowest effective dose needed to maintain adequate asthma control should be used.It is not known whether fluticasone propionate or Formoterol fumarate are excreted in human breast milk. A risk to the suckling child cannot be excluded. Therefore, a decision must be made whether to discontinue breastfeeding or to discontinue/abstain from this inhaler therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Warnings & Precaution
The management of asthma should normally follow a stepwise program and patients’ responses should be monitored clinically and by lung function tests.This inhaler should not be used to treat acute asthma symptoms for which a fast and short-acting bronchodilator is required. Patients should be advised to have their medicine to be used for relief in an acute asthma attack available at all times. The prophylactic use of this inhaler in exercise-induced asthma has not been studied. For such use, a separate rapid-acting bronchodilator should be considered. Patients should be reminded to take their inhaler maintenance dose as prescribed, even when asymptomatic. Patients should not be initiated on this inhaler during an exacerbation, or if they have significantly worsening or acutely deteriorating asthma. Serious asthma-related adverse events and exacerbations may occur during treatment with this inhaler. Patients should be asked to continue treatment but to seek medical advice if asthma symptoms remain uncontrolled or worsen after initiation on this inhaler.
How to store Flomyst® F
Do not store above 30°C. Do not refrigerate or freeze. If the inhaler is exposed to freezing conditions, then the patient must be advised to allow the inhaler to warm at room temperature for 30 minutes then re-prime the inhaler. The canister contains a pressurised liquid. Do not expose to temperatures higher than 50°C. Do not puncture, break or burn, even when apparently empty. Keep away from eyes. Keep away from children.