Exephin(Ceftriaxone)
Therapeutic Group: Anti Bacterial
Presentation
Exephin 250 mg IV injection: Each vial contains Ceftriaxone 250 mg (as sterile Ceftriaxone Sodium for injection USP).
Exephin 250 mg IM injection: Each vial contains Ceftriaxone 250 mg (as sterile Ceftriaxone Sodium for injection USP).
Exephin 500 mg IV injection: Each vial contains Ceftriaxone 500 mg (as sterile Ceftriaxone Sodium for injection USP).
Exephin 500 mg IM injection: Each vial contains Ceftriaxone 500 mg (as sterile Ceftriaxone Sodium for injection USP).
Exephin 1 g IV injection: Each vial contains Ceftriaxone 1 g (as sterile Ceftriaxone Sodium for injection USP).
Exephin 1 g IM injection: Each vial contains Ceftriaxone 1 g (as sterile Ceftriaxone Sodium for injection USP).
Exephin 2 g IV injection: Each vial contains Ceftriaxone 2 g (as sterile Ceftriaxone Sodium for injection USP).
Description
Exephin is a sterile, semisynthetic, broad-spectrum, 3rd generation cephalosporin antibiotic for intravenous or intramuscular administration. The bactericidal activity of Ceftriaxone results from inhibition of cell wall synthesis. Ceftriaxone has a high degree of stability in the presence of beta-lactamases both penicillinases and cephalosporinases of gram-negative and gram- positive bacteria
Indications
Exephin is indicated for the treatment of the following infections when caused by susceptible organisms:
Lower Respiratory Tract Infections caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, E. coli, Enterobacter aerogenes, Proteus mirabilis, Serratia marcescens.
Acute Bacterial Otitis Media caused by Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase producing strains), Moraxella catarrhalis (including beta-lactamase producing strains).
Skin and Skin Structure Infections caused by Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Viridans group streptococci, E. coli, Enterobacter cloacae, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii, Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter calcoaceticus, Bacteroides fragilis, Peptostreptococcus species.
Urinary Tract Infections (complicated and uncomplicated) caused by E. coli, Proteus mirabilis, Proteus vulgaris, Morganella morganii, Klebsiella pneumoniae.
Uncomplicated Gonorrhea (cervical, urethral, pharyngial and rectal) caused by Neisseria gonorrhoeae, including both penicillinase- and nonpenicillinase-producing strains, and pharyngeal gonorrhea caused by nonpenicillinase-producing strains of Neisseria gonorrhoeae.
Pelvic Inflammatory Disease caused by Neisseria gonorrhoeae.
Bacterial Septicemia caused by Staphylococcus aureus, Streptococcus pneumoniae, E. coli, Haemophilus influenzae, Klebsiella pneumoniae.
Bone and Joint Infections caused by Staphylococcus aureus, Streptococcus pneumoniae, E. coli, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter species.
Intra-abdominal Infections caused by E. coli, Klebsiella pneumoniae, Bacteroides fragilis, Clostridium species, Peptostreptococcus species.
Meningitis caused by Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae. Exephin has also been used successfully in a limited number of cases of meningitis and shunt infection caused by Staphylococcus epidermidis and E. coli.
Surgical Prophylaxis: The preoperative administration of a single 1 gm dose of Exephin may reduce the incidence of postoperative infections in patients undergoing surgical procedures classified as contaminated or potentially contaminated.
Dosage & Administration
Generally, Exephin should be taken once or equally devided twice a day for 4-14 days. Exephin therapy should be continued for at least 2 days after the sign and symptoms of infection have disappeared.
The usual duration of therapy is 4 to 14 days; in complicated infections longer therapy may be required. No dosage adjustment is required for patients with renal or hepatic impairment.
Side Effects
Generally Ceftriaxone is well tolerated. However, few side effects including nausea, vomiting, diarrhea, dizziness and fever may occur.
Precautions
Ceftriaxone should be administered with caution to individuals with a history of gastrointestinal disease, particularly colitis.
Use in Pregnancy & Lactation
Pregnancy: The safety of Ceftriaxone in the treatment of infections during pregnancy has not been established. Ceftriaxone should only be used during pregnancy if the likely benefit outweighs the potential risk to the fetus and/or the mother.
Lactation: Ceftriaxone is excreted in breast milk at low concentrations. Therefore, caution should be exercised when Ceftriaxone is administered to a nursing mother.
Over Dose
There is no specific antidote. Treatment of over dosage should be symptomatic.
Storage
Do not store above 30°C. Keep away from light and out of the reach of children.
Commercial Pack
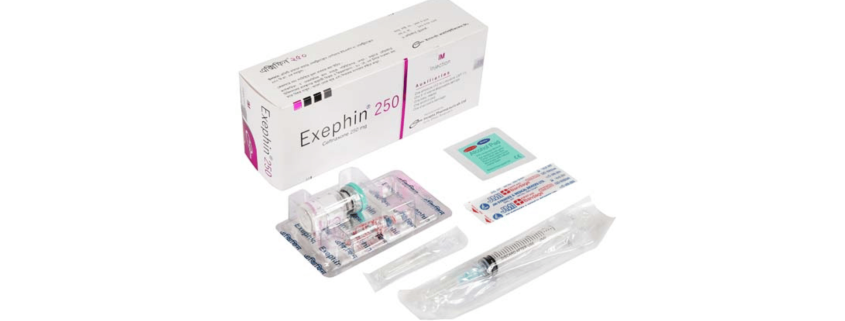
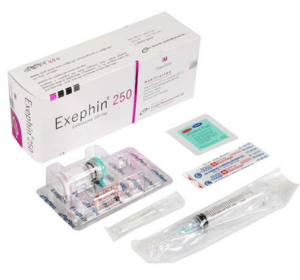
Exephin 250 mg IV injection: Each box contains one vial of 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), one ampoule of 5 ml Water for Injection BP, one sterile disposable syringe (5 ml) and a butterfly needle.
Exephin 250 mg IM injection: Each box contains one vial of 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), one ampoule of 2 ml Lidocaine Hydrochloride 1% USP, one sterile disposable syringe (5 ml) and a baby needle.
Exephin 500 mg IV injection: Each box contains one vial of 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), one ampoule of 5 ml Water for Injection BP, one sterile disposable syringe (5 ml) and a butterfly needle.
Exephin 500 mg IM injection: Each box contains one vial of 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), one ampoule of 2 ml Lidocaine Hydrochloride 1% USP, one sterile disposable syringe (5 ml) and a baby needle.
Exephin 1 g IV injection: Each box contains one vial of 1 g Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), one ampoule of 10 ml Water for Injection BP and one sterile disposable syringe (10 ml).
Exephin 1 g IM injection: Each box contains one vial of 1 g Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), one ampoule of 3.5 ml Lidocaine Hydrochloride 1% USP and one sterile disposable syringe (5 ml).
Exephin 2 g IV injection: Each box contains one vial of 2 g Ceftriaxone (as sterile Ceftriaxone Sodium for injection USP), two ampoules of 10 ml Water for Injection BP, one sterile disposable syringe (20 ml) and a butterfly needle.
Others
Use the solution immediately after reconstitution of powder.