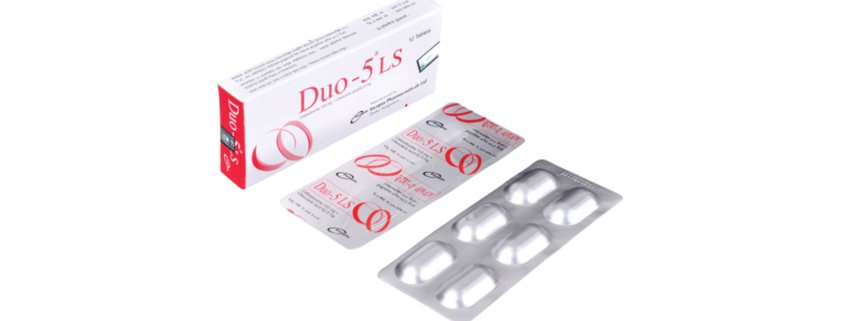
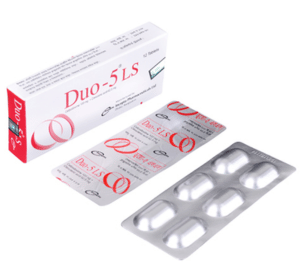
Duo-5(Cefpodoxime+ Clavulanic acid)
Therapeutic Group: Anti Bacterial
Presentation
Duo-5 LS: Each film coated tablet contains Cefpodoxime 100 mg as Cefpodoxime proxetil USP and Clavulanic acid 62.5 mg as diluted potassium Clavulanate BP.
Duo-5 : Each film coated tablet contains Cefpodoxime 200 mg as Cefpodoxime proxetil USP and Clavulanic acid 125 mg as diluted potassium Clavulanate BP.
Description
Cefpodoxime-Clavulanic acid is a combination of two drugs and is effective against multiple infection types. Clavulanic acid component protects degeneration of cefpodoxime in presence of beta-lactamase enzymes and increases the antibiotic spectrum.
Clavulanic acid in cefpodoxime-Clavulanic preparation prevents the resistance to Cefpodoxime that may increase with continuous usage of the drug. It has shown effectiveness against multiple Gram-positive and Gram-negative bacteria and is generally well tolerated.
Indications
URTIs
-Pharyngitis
-Tonsillitis
LRTIs
-Acute exacerbations of chronic bronchitis
-Acute community acquired pneumonia
SSTIs
UTIs
-Cystitis
-Uncomplicated urinary tract infections
Enteric fever
General gonorrhea and rectal gonococcal infections.
Dosage & Administration
Pharyngitis or tonsillitis:- (Duo-5 LS) 100 mg 12 hourly for 5 to 10 days
Uncomplicated urinary tract infections: (Duo-5 LS) 100 mg 12 hourly for 7 days
Complicated urinary tract infections: (Duo-5) 200 mg 12 hourly for7 days
Acute community acquired pneumonia: (Duo-5) 200 mg 12 hourly for 14 days
Acute bacterial exacerbations of chronic bronchitis: (Duo-5) 200 mg 12 hourly for 10 days
Uncomplicated gonorrhea and rectal gonococcal infections: (Duo-5) single dose
Skin and skin structure infections: (Duo-5) 200-400 mg 12 hourly for 7 to 14 days
Acute maxillary sinusitis: (Duo-5) 200 mg 12 hourly for 10 days
Enteric fever: (Duo-5) 200 mg 12 hourly for 7 to 14 days
Patients with renal dysfunction: For patients with severe renal impairment (< 30 ml/min creatinine clearance), the dosing intervals should be increased to 24 hourly. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
Patients with cirrhosis: Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) is similar to those in healthy subjects. Dose adjustment is not necessary in this population.
Side Effects
It has very few side effects. The side effects include diarrhoea, nausea, skin and vaginal fungal infection, vulvo-vaginal infections, abdominal pain and headache.
Precautions
Cross hypersensitivity in penicillin sensitive patients, leading to serious acute hypersensitivity reactions may need treatment with epinephrine along with other emergency measures such as intravenous fluids, oxygen, airway management and intravenous antihistamine, as clinically indicated.
Use in Pregnancy & Lactation
Pregnancy
Cefpodoxime is pregnancy category B. And Clavulanic acid is pregnancy category B.
Drug Interaction
Probenecid: Renal excretion of Cefpodoxime proxetil was inhibited by Probenecid and resulted in an approximately 31% increase in AUC.
Nephrotoxic drugs: Close monitoring of renal function is advised when Cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential.
Commercial Pack
Duo-5 LS: Each Box containing 2 Alu-Alu blister strips of 6 tablets.
Duo-5 : Each Box containing 3 Alu-Alu blister strips of 4 tablets.