COXPAR(Parecoxib Sodium)
Therapeutic Group: Nonsteroidal Anti-Inflammatory
Presentation
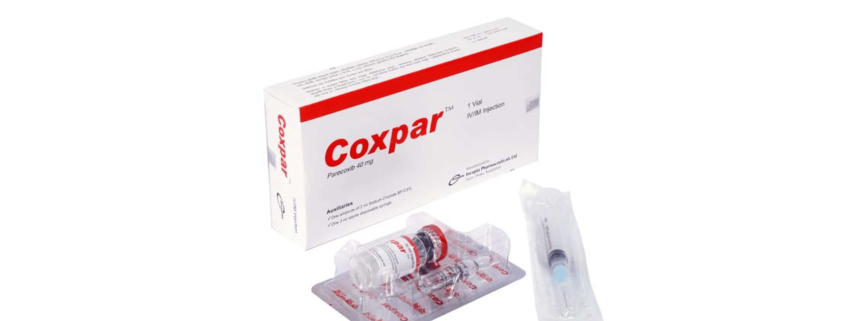
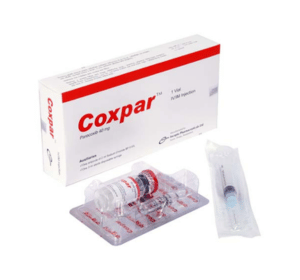
COXPARTM (IV/IM) Injection: Each vial contains Parecoxib Sodium INN equivalent to Parecoxib 40 mg.
Description
COXPARTM (IV/IM) Injection is available as lyophilised powder. Parecoxib sodium is rapidly converted to valdecoxib. The mechanism of action of valdecoxib is by inhibition of cyclooxygenase-2 (COX-2)-mediated prostaglandin synthesis. At therapeutic plasma concentrations in humans, valdecoxib does not inhibit cyclooxygenase-1 (COX-1). By inhibition of both peripheral and central COX-2, valdecoxib reduces the production of prostaglandins that are important mediators of pain and inflammation. COXPARTM Injection is not potential for dependence, sedation or respiratory depression seen with opioid analgesic agents.
Indications
COXPARTM is indicated for the management of post-operative pain.
Dosage & Administration
The usual recommended dose is a single 40 mg dose administered intravenously (IV) or intramuscularly (IM), followed every 6 to 12 hours by 20 mg or 40 mg as required, not to exceed 80 mg/day. The IV bolus injection may be given rapidly and directly into a vein or into an existing IV line. The IM injection should be given slowly and deeply into the muscle.
Elderly
No dosage adjustment is generally necessary. However, for elderly female patients weighing less than 50 kg, the recommended dose of COXPARTM Injection is 20 mg.
Hepatic Impairment
No dosage adjustment is generally necessary in patients with mild hepatic impairment. Introduce COXPARTM Injection with caution and at half the usual recommended dose in patients with moderate hepatic impairment. There is no clinical experience in patients with severe hepatic impairment.
Renal Impairment
On the basis of pharmacokinetics, no dosage adjustment is necessary in patients with mild to moderate (creatinine clearance of 30-80 mL/min) or severe (creatinine clearance <30 mL/min) renal impairment. However, caution should be observed in patients with severe renal impairment or patients who may be predisposed to fluid retention.
Children
COXPARTM Injection has not been studied in patients under 18 years old.
Incompatibilities
This medicinal product must not be mixed with other medicinal products and should be reconstituted only with sodium chloride solution (0.9% w/v).
Instructions for Use
Reconstitute COXPARTM Injection with 2 mL (40 mg vials) sodium chloride solution (0.9% w/v) using aseptic technique. After reconstitution, COXPARTM Injection should be inspected visually for particulate matter and discoloration prior to administration. The solution should not be used if discolored or cloudy or if particulate matter is observed. To reduce microbiological hazard, use as soon as practicable after reconstitution. The reconstituted product should not be stored in a refrigerator or freezer.
Precautions
Parecoxib should be used with caution in patients with hypertension, hyperlipidaemia, diabetes mellitus, sulfonamide allergy, GI Ulceration, Bleeding, hepatic dysfunction, severe hypotension and pre-existing asthma. The use of an ACE inhibiting medicine (ACE-inhibitor or angiotensin receptor antagonist), and an anti-inflammatory drug (NSAID or COX-2 inhibitor) and a thiazide diuretic at the same time, increases the risk of renal impairment.
Use in Pregnancy & Lactation
Pregnancy Category C. The use of COXPARTM injection during the first trimester and third trimesters should be avoided.
Parecoxib are reported to be transferred into the breast milk of lactating women. Because of the potential for adverse effects in nursing infants from COXPARTM injection, breast feeding should be discontinued during treatment.
Drug Interaction
Parecoxib have interactions with the following class of drugs:
Aspirin, Methotrexate, ACE-inhibitors, Injectable Anaesthetics, Inhalation Anaesthetics, Glibenclamide, Anticonvulsants, Ketoconazole, Fluconazole, Dextromethorphan, Lithium and Anticoagulants.
Over Dose
There are no specific antidotes. Patients should be managed by symptomatic and supportive care following an overdose.
Storage
Do not store above 30 C. Keep away from light and out of the reach of children.
Commercial Pack
COXPARTM (IV/IM) Injection: Each box contains one vial of Parecoxib sodium, one ampoule of 2ml 0.9% NaCl and one sterile disposable syringe (3ml).