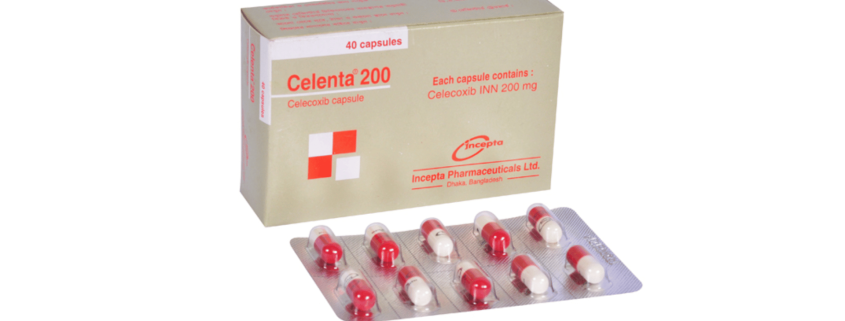
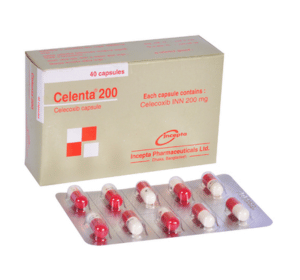
Celenta(Celecoxib)
Therapeutic Group: Analgesic, Anti Inflammatory
Presentation
Celenta 100: Each capsule contains Celecoxib INN 100 mg.
Celenta 200: Each capsule contains Celecoxib INN 200 mg.
Description
Celenta is a non-steroidal anti-inflammatory agent (specific COX-2 inhibitor) having marked anti-inflammatory, analgesic and antipyretic activities with lower incidence of gastrointestinal adverse effects.
Indications
Celenta capsule is indicated for the relief of pain and inflammation of osteoarthritis and adult rheumatoid arthritis. Celenta is also indicated to reduce the number of adenomatous colorectal polyps in familial adenomatous polyposis (FAP); as an adjunct to usual care (e.g. endoscopic surveillance, surgery).
Dosage & Administration
For osteoarthritis and rheumatoid arthritis, the lowest dose of Celenta should be sought for each patient. These doses can be given without regard to timing of meals.
Osteoarthritis: For relief of the signs and symptoms of osteoarthritis the recommended oral dose is 200 mg per day as a single dose or as 100 mg twice daily. Rheumatoid arthritis: For relief of the signs and symptoms of rheumatoid arthiritis the recommended oral dose is 100-200 mg twice daily. Familial adenomatous polyposis: Usual medical care for FAP patients should be continued while on Celenta capsules. To reduce the number of adenomatous colorectal polyps in patients with FAP, the recommended oral dose is 400 mg twice daily to be taken with food. Hepatic insufficiency: The daily recommended dose of Celenta capsules in patients with moderate hepatic impairment should be reduced approximately by 50%.
Side Effects
Gastrointestinal side effects include abdominal pain, diarrhoea, dyspepsia, flatulence and nausea. Central nervous system side effects include dizziness, headache and insomnia. Other side effects include upper respiratory tract infection, skin rash, back pain and peripheral edema.
Precautions
Celecoxib cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids. Celecoxib should be prescribed with extreme caution in patients with a prior history of G.I. ulcer-disease or G.I. bleeding, hepatic and renal insufficiency, heart failure, those taking diuretics and ACE inhibitors, pre-existing asthma, elderly patients.
Use in Pregnancy & Lactation
Celecoxib should be used during pregnancy only if the potential benefit justifies the potential risk to fetus. But in late pregnancy Celecoxib should be avoided because it may cause premature closure of ductus arteriosus. It is not known whether Celecoxib is excreted in human milk. Because many drugs are excreted in human milk and because of potential for serious adverse reactions in nursing infants from Celecoxib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Over Dose
Patients should be managed by symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. No information is available regarding the removal of Celecoxib by hemodialysis but based on its high degree of plasma protein binding (>97%) dialysis is unlikely to be useful in overdose. Emesis and/or activated charcoal and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose. Forced diuresis, alkalinization of urine, hemodialysis or hemoperfusion may not be useful due to high protein binding.
Commercial Pack
Celenta 100: Box containing 5 blister strips of 10 Capsules.
Celenta 200: Box containing 4 blister strips of 10 Capsules.