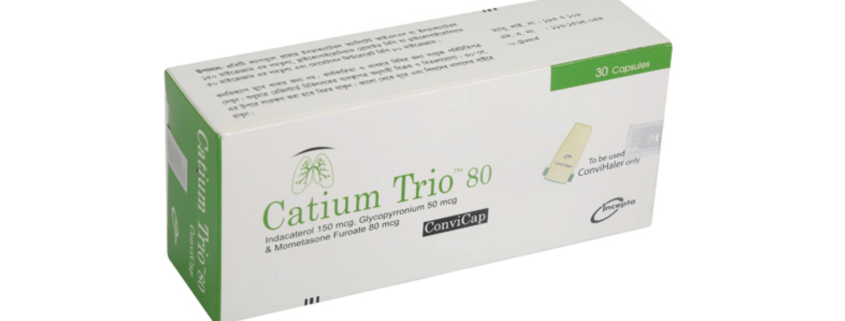
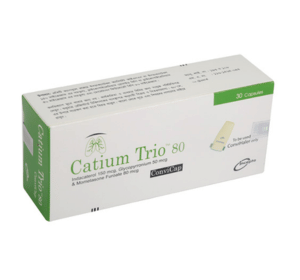
Catium Trio ConviCap(Indacaterol, Glycopyrronium & Mometasone Furoate)
Therapeutic Group: Long acting Selective Beta-2 Adrenoceptor Stimulants
Presentation
Catium Trio 80 ConviCap: Each capsule contains Indacaterol Acetate INN equivalent to Indacaterol 150 mcg, Glycopyrronium Bromide BP equivalent to Glycopyrronium 50 mcg and Mometasone Furoate BP 80 mcg.
Catium Trio 160 ConviCap: Each capsule contains Indacaterol Acetate INN equivalent to Indacaterol 150 mcg, Glycopyrronium Bromide BP equivalent to Glycopyrronium 50 mcg and Mometasone Furoate BP 160 mcg.
Description
This medicinal product is a combination of Indacaterol, a long-acting beta2-adrenergic agonist (LABA), Glycopyrronium, a long-acting muscarinic receptor antagonist (LAMA) and Mometasone Furoate, an inhaled synthetic corticosteroid (ICS). The pharmacological effects of beta2-adrenoceptor agonists, including indacaterol, are at least in part attributable to increased cyclic-3’, 5’-adenosine monophosphate (cyclic AMP) levels, which cause relaxation of bronchial smooth muscle. Glycopyrronium works by blocking the bronchoconstrictor action of acetylcholine on airway smooth muscle cells, thereby dilating the airways. Glycopyrronium bromide is a high-affinity muscarinic receptor antagonist. It demonstrated 4- to 5-fold selectivity for the human M3 and M1 receptors over the human M2 receptor in competition binding studies. Mometasone Furoate is a synthetic corticosteroid with high affinity for glucocorticoid receptors and local anti-inflammatory properties.
Indications
It is indicated as a maintenance treatment of Asthma in adult patients not adequately controlled with a maintenance combination of a long-acting beta2-agonist and a high dose of an inhaled corticosteroid who experienced one or more asthma exacerbations in the previous year.
Dosage & Administration
The maximum recommended dose is one capsule to be inhaled once daily.
Side Effects
Common side effects may include difficulty breathing or swallowing, swelling of the tongue, lips or face, skin rash, itching and hives. Other side effects include sore throat, runny nose, sudden difficulty breathing and feeling of tightness in chest with wheezing or coughing.
Precautions
This medicinal product should not be used to treat acute Asthma symptoms, including acute episodes of bronchospasm, for which a short-acting bronchodilator is required. Increasing use of short-acting bronchodilators to relieve symptoms indicates deterioration of control and patients should be reviewed by a physician.
Use in Pregnancy & Lactation
Pregnancy: This medicinal product should only be used during pregnancy if the expected benefit to the patient justifies the potential risk to the foetus.
Lactation: A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from therapy, taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Drug Interaction
No specific interaction studies were conducted with Indacaterol/Glycopyrronium/Mometasone Furoate. Information on the potential for interactions is based on the potential for each of the monotherapy components.
Over Dose
The maximum recommended dose is one capsule to be inhaled once daily.
Storage
Do not store above 30 °C.
Keep away from light and out of the reach of children.
Protect from freezing.
Insert the ConviCap in the ConviHaler just prior to use to protect from deterioration by moisture.
Commercial Pack
Catium TrioTM 80 ConviCap: Each box contains 5 Alu-Alu blister strips of 6 capsules. Catium TrioTM 160 ConviCap: Each box contains 5 Alu-Alu blister strips of 6 capsules.