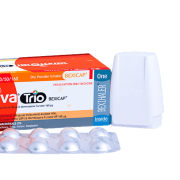
Bexitrol F Maxhaler

Generic Name: Salmeterol and Fluticasone Propionate
Dosage Form: Inhalation Powder, Pre-dispensed
TG Name: Respiratory
What Bexitrol® F Maxhaler® is and what it is used for ?
Bexitrol® F Maxhaler® is a Multi dose Dry Powder Inhaler, combination of Salmeterol Xinafoate BP and Fluticasone Propionate BP. Salmeterol Xinafoate is a selective, long acting β2 agonist used in the treatment of asthma and other forms of diffuse airways obstruction. Fluticasone Propionate is a corticosteroid with mainly glucocorticoid activity. Fluticasone Propionate is stated to exert a topical effect on the lungs without systemic effects at usual dose.
Maxhaler® is a moulded plastic device containing a foil strip with 60 regularly placed blisters containing pre-dispensed inhalation powder.Bexitrol® F is indicated in the regular treatment of asthma and COPD.
How to take Bexitrol® F Maxhaler® ?
Patients should be made aware that Bexitrol® F Maxhaler® must be used daily for optimum benefit, even when asymptomatic.
Asthma
Adults and Adolescents (12 years and older): Bexitrol® F 50/100, 50/250, 50/500 Maxhaler®: One Inhalation twice daily
Children (4 years and older): Bexitrol® F 50/100 Maxhaler®: One Inhalation twice daily
The maximum licensed dose of fluticasone propionate delivered by Bexitrol® F Maxhaler® in children is 100 mg twice daily.
There are no data available for use of Bexitrol® F in children aged under 4 years.
COPD
Adults: Bexitrol® F 50/500 Maxhaler®: One Inhalation twice daily
Using the Maxhaler®: This is a patient friendly, ready to use and easy to grip device. Use as per instructions for use.
Possible side effects
The following side effects were commonly reported: Candidiasis of the mouth and throat, Pneumonia (in COPD patients), Bronchitis, Hypokalaemia, Headache, Nasopharyngitis, Throat irritation, Hoarseness/dysphonia, Sinusitis, Contusions, Muscle cramps, Traumatic fractures, Arthralgia and Myalgia.
Pregnancy and Lactation
Administration of Bexitrol® F Maxhaler® to pregnant women should only be considered if the expected benefit to the mother is greater than any possible risk to the fetus. The lowest effective dose of fluticasone propionate needed to maintain adequate asthma control should be used in the treatment of pregnant women.
It is unknown whether Salmeterol and fluticasone propionate/metabolites are excreted in human milk. Studies have shown that Salmeterol and fluticasone propionate, and their metabolites, are excreted into the milk of lactating rats. A risk to breastfeed newborns/infants cannot be excluded. A decision must be made whether to discontinue breastfeeding or to discontinue Bexitrol® F therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Warnings & Precaution
Bexitrol® F Maxhaler® should not be used to treat acute asthma symptoms for which a fast- and short-acting bronchodilator is required. Patients should be advised to have their inhaler to be used for relief in an acute asthma attack available at all times.
Patients should not be initiated on Bexitrol® F Maxhaler® during an exacerbation, or if they have significantly worsening or acutely deteriorating asthma.
How to store Flomyst® F
Avoid storage in direct sunlight or heat. Do not store above 30°C. Store in a dry place. Keep away from children. Store your Maxhaler® in Carry-bag and always keep your information leaflet inside.
Special precautions for disposal and other handling of Bexitrol® F Maxhaler®
Do not open until you ready to inhale it because after each opening of mouthpiece cover one dose is opened and your device will be clogged if it is not inhaled. If you accidentally open mouthpiece cover/ lever, make sure to gently shaking by holding mouthpiece downward to remove extra powder. The Maxhaler® releases a powder which is inhaled into the lungs. A dose indicator on the Maxhaler® indicates the number of doses left.