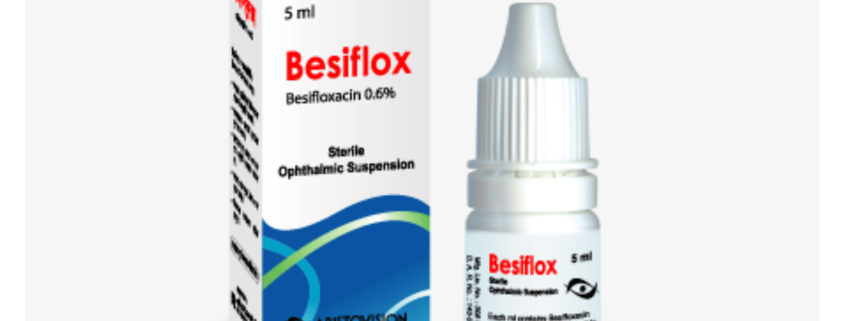
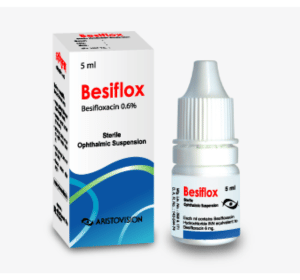
Besiflox (Besifloxacin Hydrochloride)
Therapeutic Group : Antibiotic
Presentation:
Besiflox Sterile Ophthalmic Suspension: Each ml contains Besifloxacin Hydrochloride equivalent to Besifloxacin INN 6 mg.
Indications:
Besiflox Sterile Ophthalmic Suspension is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms: Aerobic Gram-Positive Bacteria: Corynebacterium pseudodiphtheriticum, Corynebacterium striatum, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus lugdunensis, Streptococcus mitis, Streptococcus pneumoniae, Streptococcus oralis, Streptococcus salivarius, CDC coryneform group G. Aerobic Gram-Negative Bacteria: Moroxella lacunata. It is also indicated for the treatment of blepharitis, surgical prophylaxis & keratitis.
Dosage & Administration:
For bacterial conjunctivitis & blepharitis: Instill 1 drop in the affected eye(s) 3 times a day for 7 days. For surgical prophylaxis: Instill 1 drop in the affected eye(s) every 10 minutes for a total 4 doses beginning 1 hour before cataract surgery. For keratitis: Instill higher doses in the affected eye(s) as per needed or directed by the physicians.
Contrainidications:
None
Warning & Precautions:
For topical ophthalmic use only and should not be injected subconjunctivally and should not be introduced directly into the anterior chamber of the eye. Prolonged use may result in overgrowth of non-susceptible organisms, including fungi like with other anti-invectives. Patients should be advised not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis.
Side effects:
Reported side effect is conjunctival redness. Other side effects are blurred vision, eye irritation, eye pain, eye pruritus and headache.
Besiflox Sterile Ophthalmic Suspension: Each plastic dropper bottle contains 5 ml sterile ophthalmic suspension.