Aroflo® HFA inhaler (Salmeterol & Fluticasone Propionate)
Description
Aroflo® HFA inhaler is a combination of Salmeteroland Fluticasone Propionate. Salmeterol is aselective, long acting ß2 agonist used in the treatment of asthma and other forms of diffuse airways obstruction. Fluticasone Propionate is corticosteroid with potent anti-inflammatory activity. Fluticasone Propionate is stated to exert a topicaleffect on the lungs without systemic effects at usual dose.
Presentation
Aroflo®-125 HFA inhaler: Each puff delivers Salmeterol Xinafoate BP equivalent to Salmeterol 25 μg and Fluticasone Propionate BP 125 μg.
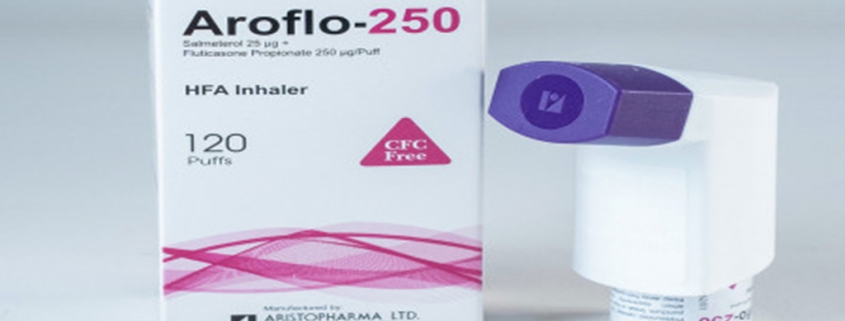
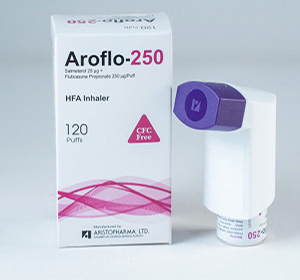
Aroflo®-250 HFA inhaler: Each puff delivers Salmeterol Xinafoate BP equivalent to Salmeterol 25 μg and Fluticasone Propionate BP 250 μg.
Indications
Aroflo® HFA inhaler is indicated in the regular treatment of asthma where use of a combination product (long-acting ß2-agonist and inhaled corticosteroid) is appropriate:
- patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short acting ß2-agonist or
- patients already adequately controlled on both inhaled corticosteroid and long-acting ß2-agonist
Dosage & Administration
Adults and adolescents 12 years and older:
2 puffs of 25 μg Salmeterol and 125 μg Fluticasone Propionate (Aroflo®-125) twice daily or 2 puffs of 25 μg Salmeterol and 250 μg Fluticasone Propionate (Aroflo®-250) twice daily.
Contrainidications
Aroflo® HFA inhaler is contraindicated in patients with hypersensitivity to any of the active substances or to the excipient of this preparation.
Warning & Precautions
Aroflo® HFA inhaler should not be used to treat acute asthma symptoms for which a fast and short acting bronchodilator is required. Aroflo® HFA inhaler should not be stopped abruptly. It should be administered with caution in patients with pulmonary tuberculosis. Aroflo® HFA inhaler should be used with caution in patients with severe cardiovascular disorders, heart rhythm abnormalities, diabetes mellitus, thyrotoxicosis, uncorrected hypokalemia or patients predisposed to low levels of serum potassium. Systemic effects may occur with any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur than with oral corticosteroids. It is important, therefore, that the patient is reviewed regularly and the dose of inhaled corticosteroid is reduced to the lowest dose at which effective control of asthma is maintained.
Side effects
As Aroflo® HFA inhaler contains Salmeterol and Fluticasone Propionate, the type and severity of adverse reactions associated with each of the compounds may be expected. There is no incidence of additional adverse events following concurrent administration of the two compounds. The pharmacological side effects of ß2-agonist treatment, such as tremor, palpitations and headache have been reported, but tend to be transient and reduce with regular therapy. Cardiac arrythmia may occur. There have been reports of arthralgia and hypersensitivity reactions, including rash, oedema and angioedema. There have been reports of oropharyngial irritation. Due to the Fluticasone Propionate component, hoarseness and candidiasis (thrush) of the mouth and throat can occur in some patients. Both hoarseness and incidence of candidiasis may be relieved by gargling with water after using the product. Symptomatic candidiasis can be treated with topical anti-fungal therapy whilst still continuing with the Aroflo® HFA inhaler. Possible systemic effects in children include adrenal suppression, growth retardation, decrease in bone mineral density.
Drug interaction
Both non-selective and selective ß-blockers should be avoided in patients with asthma, unless there are compelling reasons for their use. Care should be taken while co-administering of CYP3A4 inhibitors (i.e. Ketoconazole, Ritonavir) & Salmeterol-Fluticasone as there is an increased risk of systemic side effects of individual component.
Use in special groups
Use in pregnancy: There are insufficient data on the use of Salmeterol& Fluticasone Propionate during pregnancy. Administration of drugs to pregnant women should only be considered if the expected benefit to the mother is greater than any possible risk to the foetus.
Use in lactation: There are no data available for human breast milk. Administration of Salmeterol & Fluticasone Propionate to women who are breast-feeding should only be considered if the expected benefit to the mother is greater than any possible risk to the child.
Special patient groups: There is no need to adjust the dose in elderly patients or in those with renal impairment. There are no data available for use of Aroflo® HFA inhaler in patients with hepatic impairment.
Packing
Aroflo®-125 HFA inhaler: Each canister contains120 puffs.
Aroflo®-250 HFA inhaler: Each canister contains60 puffs.
Aroflo®-250 HFA inhaler: Each canister contains120 puffs.