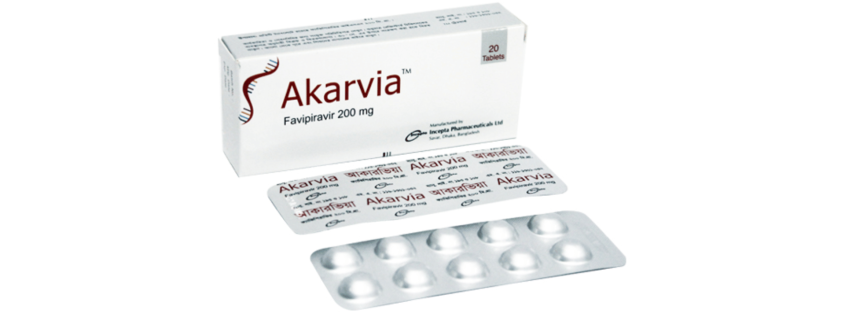
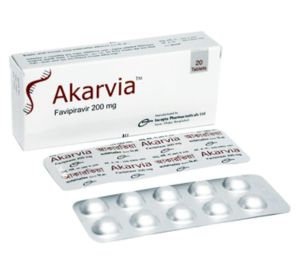
Akarvia(Favipiravir )
Therapeutic Group: Anti Viral
Presentation
Each tablet contains Favipiravir INN 200 mg.
Description
Favipiravir is an anti-influenza drug. The antiviral targets RNA dependent RNA polymerase (RdRp) enzymes, which are necessary for transcription and replication of viral genomes.
Indications
Favipiravir is approved to treat cases of influenza. It has been investigated to treat novel viruses including Ebola.
Dosage & Administration
Influenza: The usual dosage of Favipiravir for adults is 1600 mg twice daily in Day 1 followed by 600 mg orally two times daily for 4 days. The total administration period will be 5 days.
Side Effects
Raised serum uric acid level, liver & kidney injury, flatulence, decreased count of WBC, neutrophil & platelet, hallucination, delusion, shock, anaphylaxis and jaundice.
Precautions
It is only considered in outbreak of re-emerging influenza viral infections and it is not effective in bacterial infections. Pediatric use: It is not indicated for administration to pediatrics.
Geriatric use: Favipiravir should be administered with care in elderly patients by monitoring their general & vital conditions.
Use in Pregnancy & Lactation
No clinical data was available for pregnant women being treated with Favipiravir. It is not known whether it is excreted in human milk. Mothers should be instructed not to breast feed if they are taking Favipiravir
Drug Interaction
NA
Over Dose
Toxicity for over dosing information regarding Favipiravir in human body is not readily available.
Withdrawal Period
NA
Storage
Do not store above 30°C. Keep away from light and out of reach of children.
Commercial Pack
Each box contains 2 blister strips of 10 tablets