Adrinor(Adrenaline)
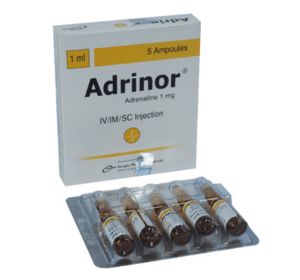
Therapeutic Group: Cardiovascular
Presentation
Adrinor Injection: Each ampoule contains Adrenaline Acid Tartrate BP equivalent to Adrenaline 1 mg in 1 ml solution for injection.
Description
Adrenaline is a direct-acting sympathomimetic agent exerting its effect on alpha and beta-adrenoceptors. The overall effect of adrenaline depends on the dose, and may be complicated by the homeostatic reflex responses. In resuscitation procedures it is used to increase the efficacy of basic life support. It is a positive cardiac inotrope. Major effects are increased systolic blood pressure by arterioral and venous vasoconstriction (alpha1 effects), reduced diastolic pressure, tachycardia and hyperglycaemia. Adrenaline is rapid in onset and with short duration. After IV infusion the half-life is approximately 5-10 minutes. It is rapidly distributed to the heart, spleen, several glandular tissues and adrenergic nerves. Adrenaline is rapidly metabolised in the liver and tissues by oxidative de-amination and O-methylation followed by reduction or by conjugation with glucuronic acid or sulphate. Up to 90% of the IV dose is excreted in the urine as metabolites. It is approximately 50% bound to plasma proteins.
Indications
Adjunctive use in the management of cardiac arrest. It is used in cardiopulmonary resuscitation. Intracardiac puncture and intramyocardial injection of adrenaline may be effective when external cardiac compression and attempts to restore the circulation by electrical defibrillation or use of a pacemaker fail. Adrenaline is a drug that leads to increased blood pressure, increased heart rate, increased air entry, increased blood glucose, stimulates cardiac activity and reduces allergic reactions by reducing inflammatory response caused by histamine. Due to these properties, it is used for the treatment of allergic and anaphylactic reactions. Adrenaline is the favored treatment for anaphylactic shock and should be administered immediately if a person begins exhibiting severe allergic reactions. Adrenaline is also used in life threatening asthma when failing ventilation and continued deterioration despite nebulizer therapy.
Dosage & Administration
Cardiac arrest:
1. Intravenous injection: 1 mg injection repeated every 2-3 minutes as necessary.
2. Endotracheal: 2-3 mg via an endotracheal tube, repeated as necessary.
3. Intracardiac injection: 0.1 to 1 mg, direct into the atrium of the heart.
4. Intraspinal use: Usual dose is 0.2 to 0.4 mg added to anesthetic spinal fluid mixture (to prolong anesthetic action by limiting absorption).
Anaphylaxis, asthma or severe bronchospasm: Adult dose is 0.25 – 0.5 mg. It may be repeated at 5 minutes intervals until perfusion and respiratory status normalizes. In case of dose dilution: 1 mg of Adrenaline to be diluted in 9 ml Normal Saline.
Children: Intravenous injection: Initially 10 mcg/kg body weight, not to exceed 250 mcg. May be repeated every 3-5 minutes if necessary. Subsequent doses should be 100 mcg/kg.
Side Effects
Anxiety, restlessness, dizziness, headache, palpitations, rapid pulse, tremors, weakness and coldness of the extremities may be reported even with small doses and especially when given in conjunction with local anaesthetics.
Precautions
The solution should not be used if it is pinkish or darker than slightly yellow or if it contains a precipitate. Adrenaline is readily destroyed by alkalies and oxidizing agents. In the latter category are Oxygen, Chlorine, Iodine, Permanganates, Chromates, Nitrites and salts of easily reducible metals, especially Iron. Adrenaline should not be mixed with Sodium bicarbonate; the solution is oxidised to adrenochrome and then forms polymers.
Special warnings and precautions for use
Administer slowly with caution to elderly patients and to patients with ischemic heart disease, hypertension, diabetes mellitus, hyperthyroidism or psychoneurosis. Use with extreme caution in patients with long-standing bronchial asthma and emphysema who have developed degenerative heart disease. Anginal pain may be induced when coronary insufficiency is present.
Use in Pregnancy & Lactation
Pregnancy Category C. It crosses the placenta and is excreted in breast milk. Adrenaline should only be used in pregnancy if the potential benefits outweigh the risks to the fetus.
Lactating mothers: It is excreted in breast milk and therefore Adrenaline is not recommended for use during lactation because of the risk of adverse effects of infants.
Drug Interaction
Use of Adrenaline with excessive doses of digitalis, mercurial diuretics or other drugs that sensitize the heart to arrhythmias is not recommended. The adverse effects of Adrenaline may be potentiated by tricyclic antidepressants; certain antihistamines; e.g, Diphenhydramine, Tripelennamine, Chlorpheniramine and L-thyroxine Sodium.
Over Dose
Symptoms: Cardiac arrhythmia leading to ventricular fibrillation, severe hypertension leading to pulmonary edema and cerebral hemorrhage.
Treatment: Combined alpha and beta-adrenergic blocking agents such as Labetalol may counteract the effects of adrenaline, or a beta-blocking agent may be used to treat any supraventricular arrhythmias and Phentolamine to control the alpha-mediated effects on the peripheral circulation. Rapidly acting vasodilators such as nitrates and Sodium Nitroprusside may also be helpful. Immediate resuscitation support must be available.
Storage
Store below 25 °C. Protect from light.
Commercial Pack
Adrinor Injection: Each commercial box contains 5 amber ampoules.