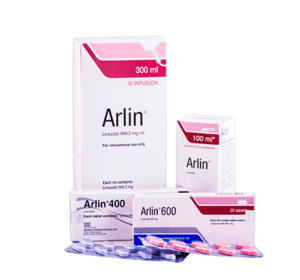
Arlin
Generic Name: Linezolid
Dosage Form: Tablet/Powder for Suspension/IV infusion
TG Name: Anti-infectives
What is Arlin®?
Arlin® contains Linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. Arlin tablets for oral administration contain 400 mg or 600 mg Linezolid as film-coated tablets. Arlin for Oral Suspension is supplied as powder for reconstitution into a suspension for oral administration. Following reconstitution, each 5 ml contains 100 mg of Linezolid. Arlin 300 ml IV infusion contains 600 mg of Linezolid.
Arlin® is used for
Arlin® is indicated for treating Complicated and uncomplicated SSTIs, Pneumonia, Bacteremia and Diabetic Foot Infections caused by susceptible gram positive bacteria.
Dosage and Administration
Adult: Complicated skin and skin structure infections, Community-acquired pneumonia including concurrent bacteremia and Nosocomial Pneumonia: 600 mg bid for 10 to 14 days. Vancomycin-resistant Enterococcus faecium infections including concurrent bacteremia: 600 mg bid for 14 to 28 days. Uncomplicated skin and skin structure infections: 400 mg bid for 10 to 14 days.
Children: Complicated skin and skin structure infections, Community-acquired pneumonia including concurrent bacteremia and Nosocomial Pneumonia: 10 mg/kg bid for 10 to 14 days. Vancomycin-resistant Enterococcus faecium infections including concurrent bacteremia: 10 mg/kg bid for 14 to 28 days. Uncomplicated skin and skin structure infections: above 5 years – 10 mg/kg bid for 10 to 14 days. Below 5 years – 10 mg/kg tid for 10 to 14 days.
Use in Special Population
Pregnancy: There are no adequate and well controlled studies in pregnant women. Arlin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether Linezolid is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Arlin is administered to a nursing woman.
Pediatric use
Can be used from day one