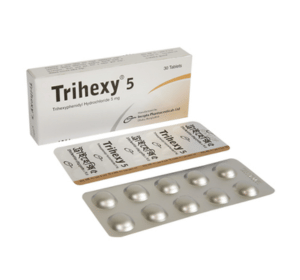
Trihexy(Trihexyphenidyl Hydrochloride)
Therapeutic Group: Drugs of Nervous System
Presentation
Trihexy 1: Each tablet contains Trihexyphenidyl Hydrochloride USP 1 mg
Trihexy 2: Each tablet contains Trihexyphenidyl Hydrochloride USP 2 mg .
Trihexy 5: Each tablet contains Trihexyphenidyl Hydrochloride USP 5 mg.
Trihexy 2 Syrup: Each 5 ml syrup contains Trihexyphenidyl Hydrochloride USP 2 mg.
Trihexy 5 Syrup: Each 5 ml syrup contains Trihexyphenidyl Hydrochloride USP 5 mg.
Description
Trihexyphenidyl exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue itself and indirectly through an inhibitory effect upon the parasympathetic nervous system.
Indications
Trihexyphenidyl HCl tablets are indicated as an adjunct treatment of all forms of parkinsonism (postencephalitic, arteriosclerotic & idiopathic). Additionally, it is indicated for the control of extrapyramidal disorders caused by central nervous system drugs such as dibenzoxazepines, phenothiazines, thioxanthenes & butyrophenones.
Dosage & Administration
Dosage should be individualized. The initial dose should be low and then increased gradually, especially in patients over 60 years of age. Whether Trihexy (trihexyphenidyl HCl) may best be given before or after meals should be determined by the way the patient reacts.
Idiopathic Parkinsonism
1 mg of Trihexy (trihexyphenidyl HCl) tablet maybe administered the first day. The dose may then be increased by 2mg increments at intervals of three to five days.
Drug-Induced Parkinsonism
Commence therapy with a single 1 mg dose increase the total daily dosage to 5-15 mg range if the extrapyramidal manifestations are not controlled.
Concomitant Use with Levodopa
When Trihexy (trihexyphenidyl HCl) is used concomitantly with levodopa, the usual dose is 3-6 mg daily.
Side Effects
Minor side effects such as dryness of the mouth, blurring of vision, dizziness, mild nausea or nervousness. Patients with arteriosclerosis or with a history of idiosyncrasy to other drugs may exhibit reactions of mental confusion, agitation, disturbed behavior, or nausea and vomiting. Potential side effects are constipation, drowsiness, urinary hesitancy or retention, pupil dilation, increased intraocular tension, vomiting and headache.
Precautions
Patients with cardiac, liver, or kidney disorders, or with hypertensioon, should closely be monitored. Since trihexyphenidyl has parasympatholytic activity, it should be used with caution in patients with glaucoma, obstructive disease of the gastrointestinal or genitourinary tracts, and in elderly males with possible prostatic hypertrophy. Trihexyphenidyl is not recommended for use in patients with tardive dyskinesia unless they have concomitant Parkinson’s disease. Abrupt withdrawal of treatment for parkinsonism may result in acute exacerbation of parkinsonism symptoms; therefore, abrupt withdrawal should be avoided.
Use in Pregnancy & Lactation
Pregnancy: Pregnancy Category C.
Nursing mothers: It is not known whether the drug is excreted in human milk and therefore trihexyphenidyl should only be used if the expected benefit to the mother outweighs the potential risk to the infant.
Drug Interaction
Cannabinoids, barbiturates, opiates, and alcohol may have additive effects with trihexyphenidyl, and thus, an abuse potential exists. Concurrent use of alcohol or other CNS depressants with trihexyphenidyl may cause increased sedative effects. It may be contraindicated in patients taking monoamine oxidase inhibitors & tricycllic antidepressants.
Over Dose
Overdosage with trihexyphenidyl produces typical central symptoms of atropine intoxication ( the central anticholinergic syndrome). Signs & symptoms are: dilated and sluggish pupils, warm, dry skin, facial flushing, decreased secretions of mouth, pharynx, nose and bronchi, foul smelling breath, tachycardia etc. Neuropsychiatric signs such as delirium, disorientation, anxiety, hallucinations etc. The condition can progress to stupor, coma, paralysis, cardiac, respiratory arrest and death.
Storage
Do not store above 30 °C. Keep away from light and out of the reach of children.
Commercial Pack
Trihexy 1: Each box contains 3 blister strips of 10 tablets.
Trihexy 2: Each box contains 5 blister strips of 10 tablets.
Trihexy 5: Each box contains 3 blister strips of 10 tablets.
Trihexy 2 Syrup: Each bottle contains 50 ml of Trihexyphenidyl Hydrochloride syrup.
Trihexy 5 Syrup: Each bottle contains 50 ml of Trihexyphenidyl Hydrochloride syrup.