Tolosa(Tocilizumab)
Therapeutic Group: Disease-modifying Antirheumatic
Presentation
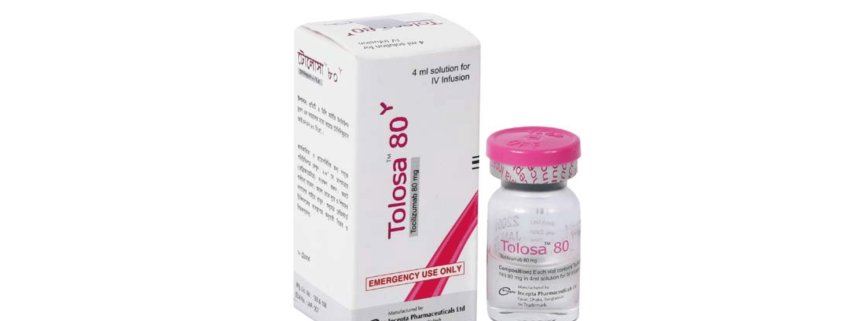
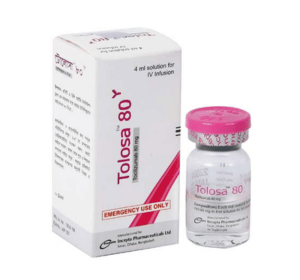
Tolosa 80 solution for IV infusion: Each vial contains Tocilizumab INN 80 mg in 4 ml solution for IV infusion.
Description
Tocilizumab is a recombinant humanized anti-human interleukin 6 (IL-6) receptor monoclonal antibody of the immunoglobulin IgG1κ (gamma 1, kappa) subclass with a typical H2L2 polypeptide structure. Each light chain and heavy chain consists of 214 and 448 amino acids, respectively. The four polypeptide chains are linked intra- and inter-molecularly by disulfide bonds. TOCILIZUMAB has a molecular weight of approximately 148 kDa. The antibody is produced in mammalian (Chinese hamster ovary) cells.
Tocilizumab injection is a sterile, clear, and colorless to pale yellow, preservative-free solution for further dilution prior to intravenous infusion with a pH of approximately 6.5. Each single-dose vial, formulated with a disodium phosphate dodecahydrate/sodium dihydrogen phosphate dehydrate buffered solution, is available at a concentration of 20 mg/mL containing 80 mg/4 mL of Tocilizumab. Each mL of solution contains polysorbate 80 (0.5 mg), sucrose (50 mg), and Water for Injection, USP.
Indications
It should not be routinely used to manage severe to critical COVID-19. It can only be used under consultant’s supervision only if following clinical and biochemical criteria are fulfilled. Discuss efficacy, possible adverse events and cost with patient or patient’s guardian before starting Tocilizumab.
1) Clinical criteria: Patients (Adult ≥ 18 years) who have been admitted to the intensive care unit (ICU) within the last 24 hours and who require invasive mechanical ventilation, noninvasive mechanical ventilation (NIV), or high-flow nasal canula (HFNC) oxygen (needing > 30L/min of oxygen and FiO2 >0.4
2) Biochemical criteria: CRP >75mg/dl
Dosage & Administration
The recommended dosage of Tocilizumab is 8 mg/kg as a single 60-minute intravenous infusion.
If clinical signs or symptoms worsen or do not improve after the first dose, one additional infusion of Tocilizumab may be administered at least 8 hours after the initial infusion.
Maximum dosage in COVID-19 patients is 800 mg per infusion.
Preparation and Administration
• For patients less than 30 kg, dilute to 50 mL in 0.9% or 0.45% Sodium Chloride Injection, USP for intravenous infusion using aseptic technique.
• For patients at or above 30 kg, dilute to 100 mL in 0.9% or 0.45% Sodium Chloride Injection, USP for intravenous infusion using aseptic technique.
• Should be administered as a single intravenous drip infusion over 1 hour; do not administer as bolus or push
Side Effects
Most common adverse reactions (incidence ≥ 3%) are constipation, anxiety, diarrhea, insomnia, hypertension and nausea
Precautions
Serious Infections – Should not be administered Tocilizumab during any other concurrent active infection
• Gastrointestinal (GI) perforation – should be used with caution in patients who may be at increased risk
• Hepatotoxicity – Tocilizumab treatment is not recommended in patients with elevated ALT or AST above 10 times the upper limit of the reference range
• Laboratory monitoring – recommended due to potential consequences of treatment-related changes in neutrophils, platelets, and liver function tests
• Hypersensitivity reactions, including anaphylaxis and death have occurred
• Live vaccines – use should be avoided with Tocilizumab
Use in Pregnancy & Lactation
Pregnancy: Tocilizumab should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus.
Lactation: Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.
Storage
Store at 2-8°C (in a refrigerator). Do not freeze. Keep away from light and out of reach of children.
Commercial Pack
Tolosa 80 solution for IV infusion: Each box contains 1 vial of 4 ml solution.