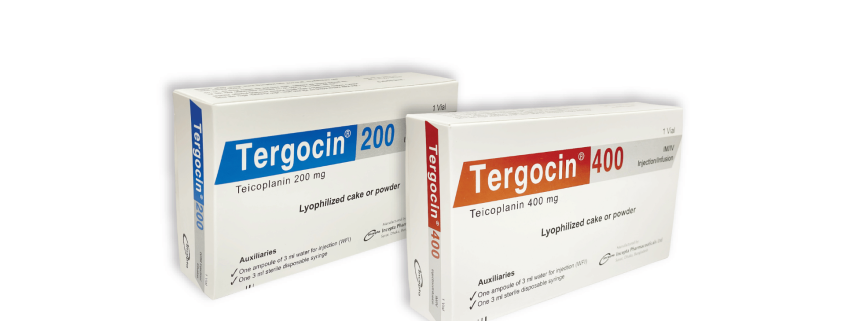
Tergocin(Teicoplanin)
Therapeutic Group: Glycopeptide antibiotic
Presentation
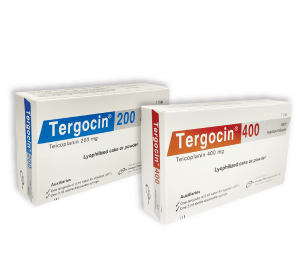
Tergocin 200 IM/IV Injection/Infusion: Each vial contains Teicoplanin BP 200 mg as lyophilized cake or powder.
Tergocin 400 IM/IV Injection/Infusion: Each vial contains Teicoplanin BP 400 mg as lyophilized cake or powder
Description
Teicoplanin is a glycopeptide antibiotic that has shown in vitro bactericidal activity against both anaerobic and aerobic Gram-positive organisms. Teicoplanin inhibits the growth of susceptible organisms by interfering with cell-wall biosynthesis at a site different from that affected by beta-lactams. It is active against staphylococci (including those resistant to methicillin and other beta-lactam antibiotics), streptococci, enterococci, Listeria monocytogenes, micrococci, group J/K corynebacteria and Gram-positive anaerobes including Clostridium difficile and peptococci
Indications
Tergocin injection is indicated for the treatment of serious infections due to staphylococci or streptococci. Following infections are treated more satisfactorily-
* Prevention of infection (usually after surgery)
* Oesteomyelitis
* Septic arthritis
* Septicaemia
* Inflammation of the lining of the heart cavity and heart
valves due to endocarditis
* Treatment of serious staphylococcal bacterial infections
* Non-cardiac bacteremia
* Dialysis associated peritonitis
* Severe infections-RTI, UTI, SSTI etc
Dosage & Administration
Adult or elderly patients with normal renal function:
* Indications-Severe infections, Loading dose- 400 mg IM/IV every 12 hours for Maintenance dose-400 mg IM/IV once daily
three doses
* Indications-Moderate infections, Loading dose- 400 mg IM/IV single dose on the first day Maintenance dose- 200 mg IM/IV once daily
*Indications-Orthopedic surgery, Loading dose- 400 mg IV at induction of anaesthesia
Child:
Age Loading dose
* Age Neonate, Loading dose- 16 mg/kg IV single dose, Maintenance dose- 8 mg/kg IV once daily
* Age-Child over 2 months, Loading dose- 10 mg/kg IM/IV every 12 hours for three doses, Maintenance dose- 6-10 mg/kg IM/IV once daily
The reconstituted solution can be administered either by intramascularly or intravenously.
Intravenously: Intravenous injection may be administered by rapid injection over 3-5 minutes, or slowly over a 30 minutes infusion by diluting with 0.9% Sodium Chloride or Hartmanns Solution or 5% Dextrose etc.
Intramascularly: An intramuscular injection of Teicoplanin should not exceed 3 ml at a single site.
The reconstituted solution can be administered either by intramascularly or intravenously.
Intravenously: Intravenous injection may be administered by rapid injection over 3-5 minutes, or slowly over a 30 minutes infusion by diluting with 0.9% Sodium Chloride or Hartmanns Solution or 5% Dextrose etc.
Intramascularly: An intramuscular injection of Teicoplanin should not exceed 3 ml at a single site.
Patients with renal impairment:
For patients with impaired renal function, reduction of dosage is not required until the fourth day of Tergocin treatment. From the fourth day of treatment IN mild renal insufficiency
Tergocin dose should be halved either by administering the initial unit dose every two days, or by administering half of this dose once a day when creatinine clearance is 40-60 ml/min.
In severe renal insufficiency
Tergocin dose should be 1/3 of the normal either by administering the initial unit dose every third day or by administering 1/3 of this dose once a day when creatinine clearance is <40 ml/min and in hemodialyzed patients. Teicoplanin is not removed by dialysis
Method of reconstitution
3 ml water for injection should be added slowly down the side wall of the vial of Tergocin 200 mg or 400 mg. The vial should be rolled gently between the palms until the powder is completely dissolved. During the rolling, we have to be cautious about the solution that it does not become foamy. The solution must not be shaken. If foam formed then it should be allowed to stand for 15 minutes for the foam to be subsided. The entire contents from the vial should be withdrawn slowly into a syringe.
Side Effects
Teicoplanin is generally well tolerated. Serious side-effects are rare. Side-effects are gastrointestinal like nausea, vomiting, diarrhea, CNS associated with urticaria, rash, anaphylactic shock as well as hearing problems like vertigo, tinnitus and vestibular disorder may occur.
Precautions
Teicoplanin should be administered with caution in patients with renal insufficiency, patients who require concurrent use of drugs which have ototoxic and/or nephrotoxic properties.
Use in Pregnancy & Lactation
There are no adequate and well-controlled studies in pregnant women about administration of Teicoplanin;
this drug should be used during pregnancy only if clearly needed.
Information about the excretion of Teicoplanin in milk is not known.
Drug Interaction
Teicoplanin should be administered with caution in patients receiving concurrent nephrotoxic or ototoxic drugs such as Aminoglycosides, Amphotericin B, Cyclosporine and Frusemide.
Storage
Do not store above 30 ºC. After reconstitution, unused portion in vial can be stored 24 hours at refrigerated temperature (2 °C to 8 °C).
Commercial Pack
Tergocin 200 IM/IV Injection/Infusion: Each box contains 1 blister pack containing 1 vial of Teicoplanin 200 mg injection, one ampoule of 3 ml water for injection (WFI) & one 3 ml sterile disposable syringe.
Tergocin 400 IM/IV Injection/Infusion: Each box contains 1 blister pack containing 1 vial of Teicoplanin 400 mg injection, one ampoule of 3 ml water for injection (WFI) & one 3 ml sterile disposable syringe.