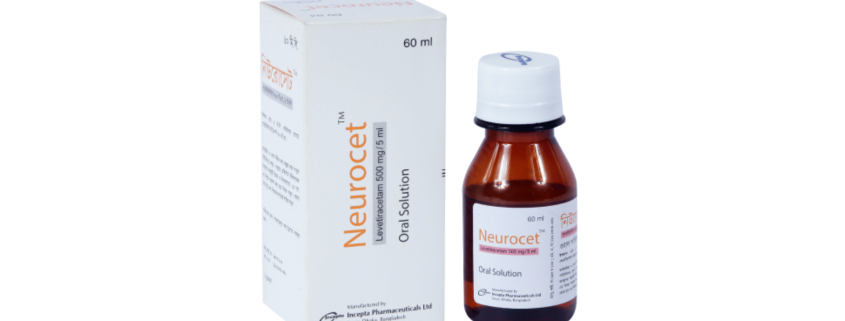
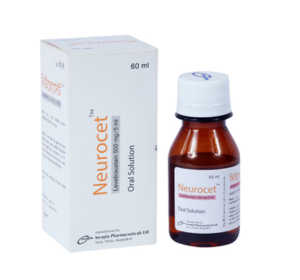
Neurocet (Levetiracetam)
Therapeutic Group: Drugs of Nervous System, Antiepileptic
Presentation
Neurocet 250 tablet: Each tablet contains Levetiracetam USP 250 mg.
Neurocet 500 tablet: Each tablet contains Levetiracetam USP 500 mg.
Neurocet XR 500 tablet: Each extended release tablet contains Levetiracetam USP 500 mg.
Neurocet XR 750 tablet: Each extended release tablet contains Levetiracetam USP 750 mg.
Neurocet XR 1 tablet: Each extended release tablet contains Levetiracetam USP 1 gm
Neurocet oral solution: Each 5 ml solution contains Levetiracetam USP 500 mg.
Neurocet 500 injection: Each 5 ml injectable solution contains Levetiracetam USP 500 mg.
Description
The precise mechanism(s) by which Levetiracetam exerts its antiepileptic effect is unknown. Levetiracetam showed only minimal activity in submaximal stimulation and in threshold tests. Protection was observed, however, against secondarily generalized activity from focal seizures induced by pilocarpine and kainic acid, two chemoconvulsants that induce seizures that mimic some features of human complex partial seizures with secondary generalization. In vitro and in vivo recordings of epileptiform activity from the hippocampus have shown that Levetiracetam inhibits burst firing without affecting normal neuronal excitability, suggesting that Levetiracetam may selectively prevent hypersynchronization of epileptiform burst firing and propagation of seizure activity.
Indications
Levetiracetam tablet, oral solution & IV injection is indicated for adjunctive therapy in the treatment of:
• Partial onset seizures in patients one month of age and older with epilepsy
• Myoclonic seizures in patients 12 years of age and older with juvenile myoclonic epilepsy
• Primary generalized tonic-clonic seizures in patients 6 years of age and older with idiopathic generalized epilepsy
Levetiracetam XR tablet is indicated as adjunctive therapy in the treatment of partial onset seizures in patients 12 years of age and older with epilepsy.
Dosage & Administration
Dosage of Levetiracetam tablet, oral solution & IV injection
Partial Onset Seizures
• 1 Month to < 6 Months: 7 mg/kg twice daily; increase by 7 mg/kg twice daily every 2 weeks to recommended dose of 21 mg/kg twice daily
• 6 Months to < 4 Years: 10 mg/kg twice daily; increase by 10 mg/kg twice daily every 2 weeks to recommended dose of 25 mg/kg twice daily
• 4 Years to < 16 Years: 10 mg/kg twice daily; increase by 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily
• Adults 16 Years and Older: 500 mg twice daily; increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily
Myoclonic Seizures
• Adults and Pediatric Patients 12 Years and Older: 500 mg twice daily; increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily
Primary Generalized Tonic-Clonic Seizures
• 6 Years to < 16 Years: 10 mg/kg twice daily; increase by 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily
• Adults 16 Years and Older: 500 mg twice daily, increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily
Dosage of Levetiracetam XR tablet
Initiate treatment with a dose of 1000 mg once daily. The once daily dosage may be adjusted in increments of 1000 mg every 2 weeks to a maximum recommended daily dose of 3000 mg/day.
Important administration instructions
Levetiracetam can be initiated with either oral or intravenous administration.
Tablet, XR tablet and oral solution
Levetiracetam tablet or XR tablet can be given orally with or without food. Prescribe the oral solution for pediatric patients with body weight 20 kg. Prescribe the oral solution or tablets for pediatric patients with body weight above 20 kg. Levetiracetam tablet or XR tablet should be swallowed whole and should not be chewed or crushed or broken. Levetiracetam XR tablet is administered once daily.
IV injection
Levetiracetam injection is for intravenous use only and must be diluted prior to administration. Levetiracetam injection (500 mg / 5 ml) should be diluted in 100 ml of compatible diluents (Sodium chloride 0.9% injection/Lactated Ringer’s injection/Dextrose 5% injection) and administered intravenously as a 15-minute IV infusion. Product with particulate matter or discoloration should not be used. Any unused portion of the Levetiracetam injection ampoule contents should be discarded.
Preparation and administration of Levetiracetam injection for Adults
• Dose: 500 mg, Withdraw volume: 5 ml (One 5 ml ampoule), Volume of diluents: 100 ml, Infusion time: 15 minutes
• Dose: 1000 mg, Withdraw volume: 10 ml (Two 5 ml ampoules), Volume of diluents: 100 ml, Infusion time: 15 minutes
• Dose: 1500 mg, Withdraw volume: 15 ml (Three 5 ml ampoules), Volume of diluents: 100 ml, Infusion time: 15 minutes
For example, to prepare a 1000 mg dose, dilute 10 ml of Levetiracetam injection in 100 ml of a compatible diluent and administer intravenously as a 15-minute infusion.
Side Effects
Most common adverse reactions (incidence ≥ 5% more than placebo) include:
• Adult patients: somnolence, asthenia, infection and dizziness
• Pediatric patients: fatigue, aggression, nasal congestion, decreased appetite, and irritability
Precautions
Levetiracetam may cause behavioral abnormalities and psychotic symptoms. Patients treated with Levetiracetam should be monitored for psychiatric signs and symptoms.
Use in Pregnancy & Lactation
Pregnancy: Pregnancy Category C.
Nursing mothers: Levetiracetam is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from Levetiracetam, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of Levetiracetam in the adjunctive treatment of partial onset seizures in pediatric patients’ age 1 month to 16 years old with epilepsy have been established.
Drug Interaction
Levetiracetam does not make interaction with Phenytoin, Valproate, Carbamazepine, Gabapentin, Lamotrigine, Phenobarbital, Primidone, Digoxin, Warfarin, Probenecid etc.
Over Dose
The highest known dose of Levetiracetam received in the clinical development program was 6000 mg/day. Other than drowsiness, there were no adverse reactions.
Commercial Pack
Neurocet 250 tablet: Each box contains 3 blister strips of 10 tablets.
Neurocet 500 tablet: Each box contains 3 blister strips of 10 tablets.
Neurocet XR 500 tablet: Each box contains 2 blister strips of 10 tablets.
Neurocet XR 750 tablet: Each box contains 2 blister strips of 10 tablets.
Neurocet XR 1 tablet: Each box contains 1 blister strip of 10 tablets.
Neurocet oral solution: Each bottle contains 60 ml of Levetiracetam oral solution.
Neurocet 500 injection: Each box contains 1 blister strip of 5 ampoules.