Maltofer(Ferric Carboxymaltose injection)
Therapeutic Group: Drugs for Hematological Disorder
Presentation
Maltofer 100: Each 2 ml solution contains Ferric carboxymaltose INN equivalent to elemental Iron 100 mg.
Maltofer 500: Each 10 ml solution contains Ferric carboxymaltose INN equivalent to elemental Iron 500 mg .
Maltofer 1 gm: Each 20 ml solution contains Ferric carboxymaltose INN equivalent to elemental Iron 1gm .
Description
Ferric carboxymaltose is a colloidal iron (III) hydroxide complex with carboxymaltose, a carbohydrate
polymer that releases iron. Ferric carboxymaltose solution is a dark brown, non-transparent and aquous
solution.
Indications
Ferric carboxymaltose is indicated for the treatment of iron deficiency anemia in adult patients:
• who have intolerance to oral iron or have had unsatisfactory response to oral iron;
• who have non-dialysis dependent chronic kidney disease.
Dosage & Administration
The cumulative dose for repletion of iron using Ferric carboxymaltose is determined based on the patient’s
body weight and haemoglobin (Hb) level and must not be exceeded. The following table (Table 1) should be
used to determine the cumulative iron dose:
Hb (g/dL):
<10 : Patients with body weight
35 kg to <70 kg 1,500 mg and Patients with body weight
≥70 kg 2,000 mg
>10: Patients with body weight
35 kg to <70 kg 1,000 mg and Patients with body weight
≥70 kg 1,500 mg
Note: A cumulative iron dose of 500 mg should not be exceeded for patients with a body weight <35 kg.
For overweight patients, a normal body weight/blood volume relationship should be assumed when
determining the iron requirement.
For patients with a Hb value ≥14 g/dL, an initial dose of 500 mg iron should be given and iron parameters
should be checked prior to repeat dosing.
Post repletion, regular assessments should be completed to ensure that iron levels are corrected and
maintained.
Maximum tolerated single dose
A single dose Ferric carboxymaltose should not exceed 1,000 mg of iron per day. Do not administer 1,000
mg of iron more than once a week.
Intravenous injection
Ferric carboxymaltose may be administered by intravenous injection using undiluted solution up to 1,000
mg iron (up to a maximum of 15 mg/kg body weight). For doses up to 200 mg iron, there is no prescribed
administration time. For doses greater than 200 and up to 500 mg iron, Maltofer should be administered at a
rate of 100 mg/min. For doses greater than 500 and up to 1,000 mg iron Maltofer should be administered
over 15 minutes.
Intravenous infusion
Ferric carboxymaltose may be administered by intravenous infusion up to a maximum single dose of 1,000
mg of iron (up to a maximum of 20 mg/kg body weight).
Method of administration
Ferric carboxymaltose must be administered only by the intravenous route: by bolus injection, or during a
haemodialysis session undiluted directly into the venous limb of the dialyser, or by infusion. In case of
infusion Maltofer must be diluted only in sterile 0.9% m/V sodium chloride solution as shown in Table 2
below.
For dilution plan of Maltofer for intravenous infusion, please see attached pdf file.
Haemodialysis-dependent chronic kidney disease
A single maximum daily injection dose of 200 mg iron should not be exceeded in haemodialysis-dependent
chronic kidney disease patients.
Side Effects
The side effects of Ferric carboxymaltose are infrequent, usually mild and generally do not cause patients to
stop treatment. The most common side effects are nausea, injection site reactions (including pain or
bruising at the injection site), and asymptomatic reductions in blood phosphorus, flushing, headache,
hypertension, dizziness, and increased alanine aminotransferase. Potentially long lasting brown staining of
skin near injection site may occur. Uncommon side effects are hypersensitivity, paraesthesia, dysgeusia,
tachycardia, hypotension, flushing, dyspnoea, vomiting, dyspepsia, abdominal pain, constipation, diarrhea,
pruritus, urticaria, erythema, rash, myalgia, back pain, arthralgia, muscle spasms, pyrexia, fatigue, chest
pain, oedema peripheral, chills, aspartate aminotransferase increased, gamma-glutamyltransferase
increased, blood lactate dehydrogenase increased and blood alkaline phosphatase increased. Very rare
side effects are anaphylactoid reactions, loss of consciousness, anxiety, phlebitis, syncope, presyncope,
bronchospasm, flatulence, angioedema, pallor face oedema, Rigors, malaise and influenza like illness.
Precautions
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been
life-threatening and fatal, have been reported in patients receiving Ferric carboxymaltose. Patients may
present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor
patients for signs and symptoms of hypersensitivity during and after Ferric carboxymaltose administration
for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Ferric
carboxymaltose when personnel and therapies are immediately available for the treatment of serious
hypersensitivity reactions. Other serious adverse reactions associated with hypersensitivity which included,
pruritus, rash, urticaria, wheezing, or hypotension may occur.
Hypertension
Transient elevations in systolic blood pressure, sometimes occurring with facial flushing, dizziness, or
nausea were observed. These elevations generally occurred immediately after dosing and resolved within
30 minutes. Monitor patients for signs and symptoms of hypertension following each Ferric carboxymaltose
administration.
Laboratory Test Alterations
In the 24 hours following administration of Ferric carboxymaltose, laboratory assays may overestimate
serum iron and transferrin bound iron by also measuring the iron in Ferric carboxymaltose.
Use in Pregnancy & Lactation
Use in paediatric population
The use of Ferric carboxymaltose has not been studied in children, and therefore is not recommended in
children under 14 years.
Use in Pregnancy and Lactation
Pregnant women
There are no data for the use of Ferric carboxymaltoxse in pregnant women. A careful risk/benefit
evaluation is required before use during pregnancy and Ferric carboxymaltoxse should not be used during
pregnancy unless clearly necessary. Animal data suggest that iron released from Ferric carboxymaltoxse
can cross the placental barrier and that its use during pregnancy may influence skeletal development in the
fetus.
If the benefit of Ferric carboxymaltoxse treatment is judged to outweigh the potential risk to the fetus, it is
recommended that treatment should be confined to the second and third trimester.
Lactating mothers
Ferric carboxymaltose is excreted in human milk which unlikely to affect the baby.
Drug Interaction
Formal drug interaction studies have not been performed with Ferric carboxymaltose.
Over Dose
Excessive dosages of Maltofer may lead to accumulation of iron in storage sites potentially leading to
hemosiderosis. Monitoring of iron parameters such as serum ferritin and transferrin saturation may assist in
recognising iron accumulation. If iron accumulation has occurred, treat according to standard medical
practice, e.g. consider the use of an iron chelator.
Storage
Keep in a cool and dry place, away from light.
Commercial Pack
Maltofer 100: Each box contains one vial of 2 ml Ferric carboxymaltose solution with one 50 ml normal
saline, one infusion set, one alcohol pad, one first aid band & one 3 ml disposable syringe.
Maltofer 500: Each box contains one vial of 10 ml Ferric carboxymaltose solution with one 100 ml normal
saline, one infusion set, one alcohol pad, one first aid band & one 10 ml disposable syringe.
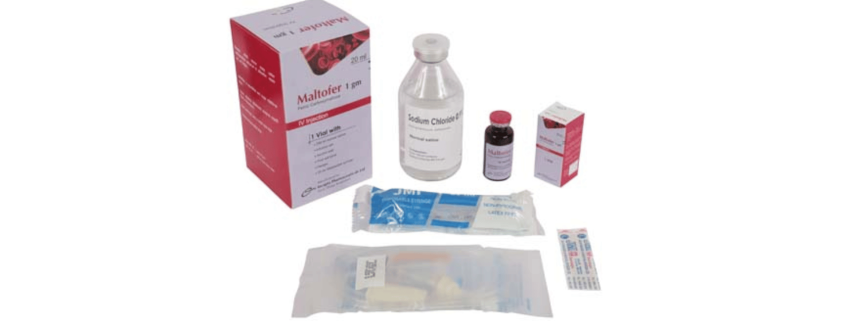
Maltofer 1 gm: Each box contains one vial of 20 ml Ferric carboxymaltose solution with one 250 ml normal
saline, one infusion set, one alcohol pad, one first aid band, hanger & one 20 ml disposable syringe.