Droslyn(Drospirenone)
Therapeutic Group: Contraceptive
Presentation
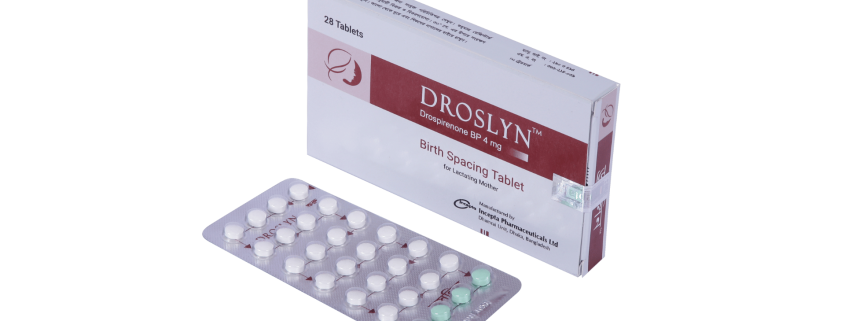
Droslyn : Each white color tablet contains Drospirenone BP 4 mg and each green color tablet is placebo.
Description
Drospirenone is a synthetic form of progesterone. It has antimineralocorticoid activity counteracting oestrogen related sodium retention. Drospirenone binds with serum albumin and does not bind with sex hormone binding globulin (SHBG) or Corticosteroid binding globulin (CBG).
The contraceptive effect of Drospirenone is achieved by Inhibition of ovulation.
Indications
Droslyn is indicated for:
• Prevention of pregnancy
Dosage & Administration
How to Take Droslyn
Droslyn (white active and green placebo tablets) is swallowed whole once a day. One tablet must be taken daily for 28 consecutive days (one white active tablet daily during the first 24 days of menstruation and one green tablet daily during the following 4 days). Tablets must be taken every day at about the same time of the day so that the interval between two tablets is always 24 hours.
1. If you have decided to take Droslyn for contraception, wait for your next menstruation begins.
2. From the first day of your menstruation, start taking the first white tablet from the right corner of the top row (with arrow mark) of your Droslyn pack.
3. Continue taking one white tablet at the same time each day for a total of 24 days and one green for next 4 days at the same time of the day that white active tablets were taken.
4. After completing the present Droslyn pack, start taking another Droslyn pack from the next day and continue taking the tablet as long as you don’t want to be pregnant.
How to start Droslyn when switching from other contraceptive methods
• From a Combined Oral Contraceptive (COC) – On the day when the new pack of the previous COC would have started.
• From Transdermal Patch, Vaginal ring and Injection – On the day when next application, insertion or injection would have been scheduled consecutively.
• From Intrauterine contraceptive and Implant – On the day of removal.
Management of missed tablets
• If one white active tablet is missed, take the missed tablet as soon as possible. Continue taking one tablet a day until the pack is finished.
• If two or more white active tablets are missed, take the last missed tablet as soon as possible. Continue one tablet a day until the pack is finished (one or more missed tablet(s) will remain in the blister pack). Additional non-hormonal contraception (such as condoms or spermicide) should be used as backup if the patient has sex within 7 days after missing tablets.
• If one or more green tablets are missed, skip the missed pill days and continue taking one tablet a day until the pack is finished.
Advice in Case of Gastrointestinal Disturbances
If vomiting or diarrhea occurs within 3-4 hours after tablet taking, the new tablet (scheduled for the next day) should be taken as soon as possible. The new tablet should be taken within 12 hours of the usual time of tablet-taking if possible. If more than two tablets are missed, the advice concerning missed tablets, including using backup non-hormonal contraception, given above is applicable.
Side Effects
The common side effectsare Hyperkalemia, Bleeding irregularities and amenorrhea. Less common side effects are Acne, Metrorrhagia, Headache, Breast pain, Weight increased, Dysmenorrhea, Nausea, Vaginal hemorrhage, Libido decreased, Breast tenderness and Irregular menstruation.
Precautions
Droslyn is contraindicated in females with conditions that predispose to hyperkalemia (e.g. renal impairment, hepatic impairment and adrenal insufficiency. Droslyn should be discontinued if arterial or venous thromboembolic event occurs. If jaundice or acute or chronic disturbances of liver function develop discontinuation of the drug should be considered. Be alert to the possibility of ectopic pregnancy in females who become pregnant or complain of lower abdominal pain while on Droslyn. Therefore, patients with diabetes may be at risk of hyperglycemia and may require additional medication adjustments. Females using Droslyn may experience intracyclic bleeding and spotting, especially during the first three months of use which may resolve over time. If bleeding persists or occurs after previously regular cycles, evaluation of pregnancy or malignancy should be done. If scheduled bleeding does not occur, consider the possibility of pregnancy. Discontinue Droslyn if females had a previous history of depression which recurs to a serious degree.
Use in Pregnancy & Lactation
Pregnancy: Incase of known or suspected pregnancy Droslyn should be discontinued
Lactation: Negligible amounts of drospirenone are excreted in the breast milk. Thus, at therapeutic doses of Droslyn, no effects on breastfed newborns/infants are anticipated. Ingeneral, no adverse effects have been found on milk production or on the health growth, or development of the infant with use of POPs.
Drug Interaction
Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the systemic concentrations of HCs and potentially diminish the effectiveness of HCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of HCs include efavirenz, phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, rifabutin, rufinamide, aprepitant. There is a potential for an increase in serum potassium concentration in females taking Drospirenone with other drugs that may increase serum potassium concentration (for example, ACE inhibitors, angiotensin-II receptor antagonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDS
Over Dose
There have been no reports of serious effects from over dosage of Droslyn. Symptoms that may occur include are nausea, vomiting, and vaginal bleeding. There are no antidotes and treatment should be to provide symptomatic support. Drospirenone is a spironolactone analogue which has antimineralocorticoid properties. Therefore, serum potassium and sodium, and evidence of metabolic acidosis, should be monitored in cases of overdose.
Storage
Do not store above 30 C. Keep away from light and out of the reach of children.
Commercial Pack
Droslyn : Each blister contains 28 tablets (24 white tablets and 4 Green color tablets). Each Droslyn strip is inserted in a Droslyn strip holder.