Megabac(Colistimethate Sodium )
Therapeutic Group: Antibiotic
Presentation
Megabac 1 M Injection: Each vial contains Colistimethate Sodium 1 MIU equivalent to 34 mg Colistin.
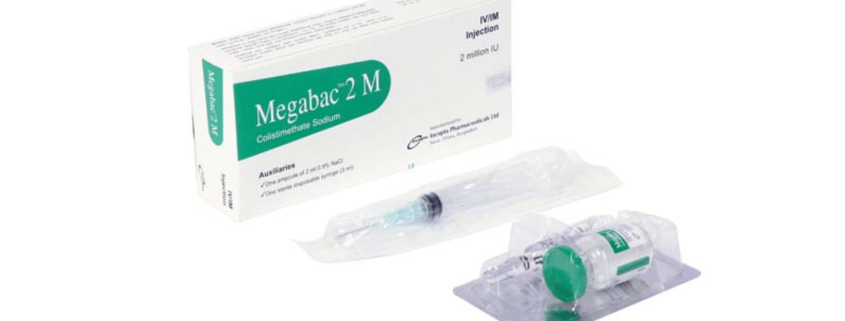
Megabac 2 M Injection: Each vial contains Colistimethate Sodium 2 MIU equivalent to 68 mg Colistin.
Description
Colistimethate sodium is a surface active agent which penetrates into and disrupts the bacterial cell membrane. It has been shown to have bactericidal activity against Aerobic gram-negative microorganisms e.g. Enterobacter aerogenes, E. coli, Klebsiella pneumoniae and Pseudomonas
Indications
Megabac is indicated for the treatment of acute or chronic infections caused by sensitive strains of certain gram-negative bacilli. It is particularly indicated in the infection caused by sensitive strains of Pseudomonas aeruginosa. Megabac is not indicated for infections due to Proteus or Neisseria. Megabac is very effective in the treatment of infections due to the following gram-negative organisms: Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa.
Dosage & Administration
Adults and adolescents:
Maintenance dose is 9MIU/day in 2-3 divided doses. In patients who are critically ill, a loading dose of 9 MIU should be administered.
Renal impairment patients:
Dose adjustments in renal impairment patients are necessary. Dose reductions are recommended for patients with creatinine clearance < 50 ml/min. Twice daily dosing is recommended.
Haemodialysis (HD) patients:
No-HD days: 2.25 MIU/day (2.2-2.3 MIU/day). HD days: 3 MIU/day (Should be given after the HD session) Twice daily dosing is recommended.
Paediatric population:
The dose should be based on lean body weight. Children ≤40kg: 75,000-150,000 IU/kg/day divided into 3 doses. Children >40kg: >150,000 IU/kg/day has been reported in children with cystic fibrosis.
Hepatic impairment patients:
There are no data in patients with hepatic impairment. Caution is advised when administering colistimethate sodium in these patients.
Administration via inhalation: Adults, adolescents and children ≥ 2 years: 1-2 MIU two to three times per day (max 6 MIU/day). Children < 2 years: 0.5-1 MIU twice daily (max 2 MIU/ day).
Side Effects
The following adverse reactions have been reported: gastrointestinal upset, tingling of extremities and tongue, slurred speech, dizziness, vertigo and paresthesia, generalized itching, urticaria and rash, fever, increased blood urea nitrogen (BUN), elevated creatinine and decreased creatinine clearance, respiratory distress and apnea, nephrotoxicity and decreased urine output.
Precautions
Megabac should be used with caution in patient with impaired renal function. When actual renal impairment is present, Megabac may be used, but the greatest caution should be exercised and the dosage should be reduced in proportion to the extent of the impairment. Megabac should be used with caution in neonates, infants and children.
Use in Pregnancy & Lactation
There are no adequate and well-controlled studies about the use of Megabac in pregnant women. Since colistimethate sodium is transferred across the placental barrier in humans, it should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether Colistimethate sodium is excreted in human breast milk. Therefore, caution should be exercised when administered to nursing women.
Drug Interaction
Megabac should not be given with certain antibiotics like- aminoglycosides and polymyxin due to report of interfere with the nerve transmission at the neuromuscular junction. It should not be given with muscle relaxants e.g., tubocurarine and other drugs including ether, succinylcholine, gallamine, decamethonium and sodium citrate. The concomitant use of Sodium Cephalothin and Megabac should be avoided.
Over Dose
Overdosage with colistimethate sodium can cause neuromuscular blockade characterized by paresthesia, lethargy, confusion, dizziness, ataxia, nystagmus, disorders of speech and apnea. Respiratory muscle paralysis may lead to apnea, respiratory arrest and death. Overdosage with the drug can also cause acute renal failure, manifested as decreased urine output and increases in serum concentrations of B.U.N and creatinine. As in any case of overdose, Colistimethate Sodium therapy should be discontinued and general supportive measures should be utilized.
Storage
Before reconstitution: Store below 30 0C
After reconstitution: Store at 2 0C to 8 0C (Do not freeze) and use within 24 hours.
Commercial Pack
Megabac 1 M injection: Each box contains one vial of Colistimethate Sodium USP equivalent to 1 million IU or 34 mg Colistin, One ampoule of 2 ml 0.9% NaCl and one sterile disposable syringe (3 ml).
Megabac 2 M injection: Each box contains one vial of Colistimethate Sodium USP equivalent to 2 million IU or 68 mg Colistin, One ampoule of 2 ml 0.9% NaCl and one sterile disposable syringe (3 ml).