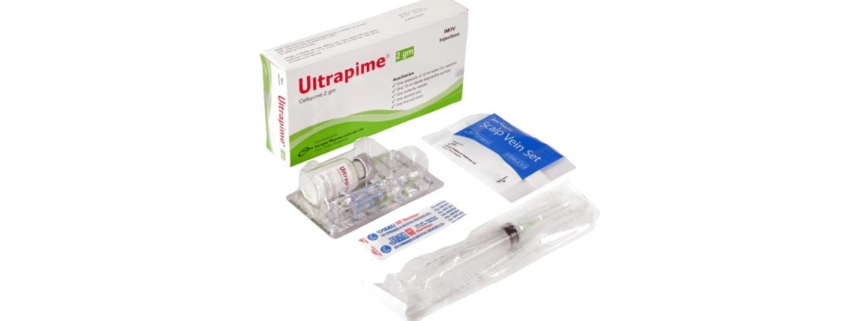
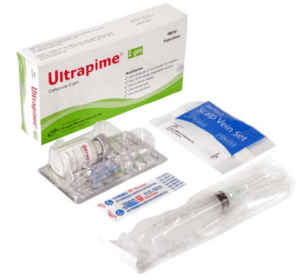
Ultrapime Injection(Cefepime)
Therapeutic Group: Anti Bacterial
Presentation
Ultrapime 500 IM/IV Injection: Each vial contains Cefepime for injection USP equivalent to Cefepime 500 mg.
Ultrapime 1 gm IM/IV Injection: Each vial contains Cefepime for injection USP equivalent to Cefepime 1 gm.
Ultrapime 2 gm IM/IV Injection: Each vial contains Cefepime for injection USP equivalent to Cefepime 2 gm.
Description
Ultrapime Injection is a preparation of Cefepime. It is a fourth generation broad-spectrum cephalosporin antibiotic. Cefepime acts by inhibition of bacterial cell wall synthesis. It is highly resistant to hydrolysis by most beta-lactamases and exhibits rapid penetration into gram-negative bacterial cells.
Cefepime has been shown to be active against most strains of the following microorganisms:
Gram-Positive Microorganisms:
Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Viridans group streptococci, Staphylococcus epidermidis, Staphylococcus saprophyticus, Staphylococcus hominis, Streptococcus agalactiae.
Gram-Negative Microorganisms:
Enterobacter, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Acinetobacter calcoaceticus, Citrobacter diversus, Citrobacter freundii, Enterobacter spp., Haemophilus influenzae (including beta-lactamase producing strains), Haemophilus parainfluenzae, Hafnia alvei, Klebsiella oxytoca, Moraxella catarrhalis (including beta-lactamase producing strains), Morganella morganii, Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Serratia marcescens, Neisseria meningitidis.
Anaerobes:
Clostridium perfringens, Mobiluncus spp.
Indications
Ultrapime Injection is indicated in the treatment of the following infections:
• Pneumonia (moderate to severe)
• Uncomplicated and Complicated Urinary Tract Infections (including pyelonephritis)
• Uncomplicated Skin and Skin Structure Infections
• Complicated Intra-abdominal Infections
• Empiric Therapy for Febrile Neutropenic Patients.
Dosage & Administration
Recommended dosage schedule for adults with normal renal function
Type of Infection Dose Frequency Duration (Days)
Moderate to severe Pneumonia 1-2 g IV q12h 10
Empiric Therapy for Febrile Neutropenic Patients 2 g IV q8h 7
Mild to moderate Uncomplicated or Complicated Urinary 0.5-1 g IV/IM q12h 7-10
Tract Infections (including pyelonephritis)
Severe Uncomplicated or Complicated Urinary 2 g IV q12h 10
Tract Infections (including pyelonephritis)
Moderate to severe Uncomplicated Skin and Skin Structure 2 g IV q12h 10
Infections
Complicated Intra-abdominal Infections 2 g IV q12h 7-10
Pediatric Patients (2 months up to 16 years)
The maximum dose for pediatric patients should not exceed the recommended adult dose.
Type of Infection Pediatric patients up to 40 kg in weight
Dose Frequency Duration
(Days)
Uncomplicated and Complicated Urinary Tract Infections 50 mg/kg q12h 7-10
(including pyelonephritis)
Uncomplicated Skin and Skin Structure Infections 50 mg/kg q12h 10
Pneumonia 50 mg/kg q12h 10
Febrile Neutropenic Patients 50 mg/kg q8h 7
Impaired Hepatic Function – No adjustment is necessary for patients with impaired hepatic function.
Impaired Renal Function – In patients with impaired renal function (creatinine clearance <60 ml/min), the dose of Cefepime should be adjusted. The recommended initial dose of Cefepime should be the same as in patients with normal renal function except in patients undergoing hemodialysis. The recommended doses of Cefepime in patients with renal insufficiency are presented in the following table:
Creatinine Clearance Recommended Maintenance Schedule
(ml/min)
>60 500 mg q12h 1g q12h 2g q12h 2g q8h
Normal recommended
dosing schedule
30-60 500 mg q24h 1g q24h 2g q24h 2g q12h
11-29 500 mg q24h 500 mg q24h 1g q24h 2g q24h
<11 250 mg q24h 250 mg q24h 500 mg q24h 1g q24h
CAPD 500 mg q48h 1g q48h 2g q48h 2g q48h
Hemodialysis 1g on day 1, then 500 mg q24h thereafter 1g q24h
Preparation of Solutions of Ultrapime Injection
Single-dose vial Administration Amount of diluent to be added
500 mg IM 1.3 ml
500 mg IV 5 ml
1 gm IM 2.4 ml
1 gm IV 10 ml
These solutions may be stored up to 24 hours at room temperature or 7 days in a refrigerator.
Ultrapime Injection is compatible at concentrations between 1 and 40 mg/ml with the following IV infusion fluids: (1) 0.9% Sodium chloride, (2) 5% and 10% Dextrose.
Side Effects
Generally Cefepime is well tolerated. However, few side-effects including rash, pruritus, urticaria, fever, headache, nausea, vomiting, diarrhea, dizziness, oral moniliasis may occur.
Precautions
In patients with impaired renal function (creatinine clearance <60 ml/min), the dose of Cefepime should be adjusted. Cefepime should be prescribed with caution in individuals with a history of gastrointestinal diseases, particularly colitis.
Use in Pregnancy & Lactation
Pregnancy: There are no adequate and well-controlled studies of Cefepime use in pregnant women. Cefepime should be used during pregnancy only if clearly needed.
Lactation: Cefepime is excreted in human breast milk in very low concentrations. Caution should be exercised when Cefepime is administered to a nursing woman.
Drug Interaction
Renal function should be monitored carefully if high doses of aminoglycosides are to be administered with Cefepime because of the increased potential of nephrotoxicity and ototoxicity of aminoglycoside antibiotics. Nephrotoxicity has been reported following concomitant administration of other cephalosporins with potent diuretics such as furosemide.
Over Dose
Patients who receive an overdose should be carefully observed and given supportive treatment. Symptoms of overdose include encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, and neuromuscular excitability.
Storage
Ultrapime Injection should be stored in a cool & dry place and protected from light.
Commercial Pack
Ultrapime 500 IM/IV Injection: Each box contains one vial of Cefepime 500 mg as Cefepime for injection USP, one ampoule of 5 ml of Water for Injection & one 5 ml sterile disposable syringe.
Ultrapime 1 gm IM/IV Injection: Each box contains one vial of Cefepime 1 gm as Cefepime for injection USP, one ampoule of 10 ml of Water for Injection & one 10 ml sterile disposable syringe.
Ultrapime 2 gm IM/IV Injection: Each box contains one vial of Cefepime 2 gm as Cefepime for injection USP, one ampoule of 10 ml of Water for Injection, one 10 ml sterile disposable syringe, one butterfly needle, one alcohol pad and one first aid band.