Cefazol(Cefazolin)
Therapeutic Group: Antibiotic
Presentation
Cefazol 500 mg Injection: Each vial contains Cefazolin Sodium USP equivalent to Cefazolin 500 mg.
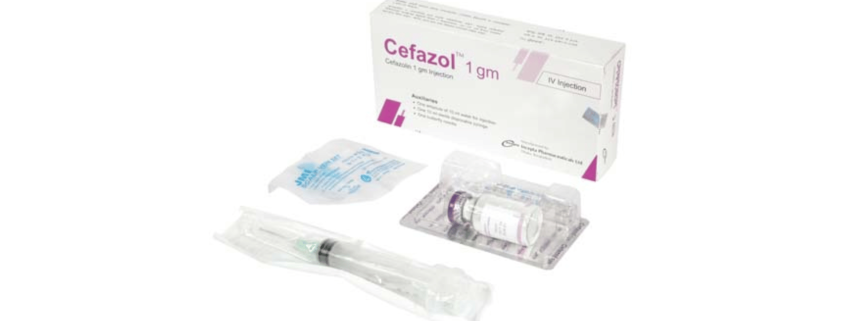
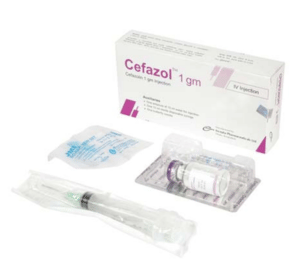
Cefazol 1 gm Injection: Each vial contains Cefazolin Sodium USP equivalent to Cefazolin 1 gm.
Description
Cefazolin is a 1st generation semi-synthetic cephalosporin antibiotic for parenteral administration. It comes in a white to yellowish powder for injection form
Indications
Cefazolin is indicated in the treatment of the following serious infections-
Respiratory Tract Infections: Due to S. pneumoniae, Klebsiella, H. influenzae, S. aureus and group A beta-hemolytic streptococci. Cefazolin is effective in the eradication of streptococci from the nasopharynx.
Urinary Tract Infections: Due to E. coli, P. mirabilis, Klebsiella species, and some strains of enterobacter and enterococci .
Skin and Skin Structure Infections: Due to S. aureus, group A beta-hemolytic streptococci, and other strains of streptococci .
Biliary Tract Infections: Due to E. coli, various strains of streptococci, P. mirabilis, Klebsiella species and S. aureus .
Bone and Joint Infections: Due to S. aureus.
Genital Infections: Due to E . coli, P . mirabilis, Klebsiella species, and some strains of enterococci .
Septicemia: Due to S. pneumoniae, S. aureus, P. mirabilis, E. coli, and Klebsiella species .
Endocarditis: Due to S. aureus and group A beta-hemolytic streptococci . Perioperative Prophylaxis: The prophylactic administration of Cefazolin for Injection preoperatively, intraoperatively, and postoperatively may reduce the incidence of certain postoperative infections in patients.
Dosage & Administration
Usual Adult Dosage
• For moderate to severe infection dose is 500 mg to 1 gm and frequency is every 6 to 8 hours
• For mild infections caused by Gram positive cocci dose is 250 mg to m00 mg and frequency is every 8 hours
• For acute uncomplicated urinary tract infection dose is 1 gm and frequency is every 12 hours
• For pneumococcal pneumonia dose is 500 gm and frequency is every 12 hours
• For severe life threatening infections dose is 1 gm to 1.5 gm and frequency is every 6 hours
Perioperative Prophylactic dose: To prevent postoperative infection, the recommended doses are: I. 1 gm IV or IM administered 1/2 hour to 1 hour prior to the start of surgery II . For lengthy surgery (e.g. 2 hours or more), 500 mg to 1 gm IV or IM during surgery III. 500 mg to 1 gm IV or IM every 6 to 8 hours for 24 hours postoperatively
Intramuscular Administration: Reconstitute vials with sterile Water for Injection. Shake well until dissolved. Cefazolin should be injected into a large muscle mass
Intravenous Administration: For direct injection reconstitution, dilute vials with approximately 10 ml Sterile Water for Injection for 500 mg and 1 gm. Inject the solution slowly over 3 to 5 minutes. For continuous infusion dilute reconstituted in 50 to 100 mL of one of the following solutions: 0.9% Sodium Chloride Injection,5% or 10% Dextrose Injection, 5% Dextrose and 0 .9% Sodium Chloride Injection.
Pediatric Dosage: In pediatric patients, a total daily dosage of 25 to 50 mg per kg of body weight, divided into 3 or 4 equal doses, is effective for most mild to moderately severe infections. Total daily dosage may be increased to 100 mg per kg of body weight for severe infections. Since safety of Cefazolin use in infants has not been established, it is not recommended
Side Effects
Swelling, redness, pain, or soreness at the injection site may occur. This medication may also rarely cause loss of appetite, nausea, vomiting, diarrhea or headache
Precautions
When Cefazolin is administered to patients with impaired renal function, a lower daily dosage is required or otherwise, seizures may occur. it should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Use in Pregnancy & Lactation
There are no adequate studies in pregnant women for this drug so this drug should be used during pregnancy only if clearly needed. Cefazolin is present in very low concentrations in the milk of nursing mothers so caution should be exercised.
Drug Interaction
Probenecid may decrease renal tubular secretion of Cefazolin, when used concurrently, resulting in increased Cefazolin blood levels
Storage
Do not store above 30 0C. Keep away from light and out of the reach of children
Commercial Pack
Cefazol 500 mg Injection: Each box contains one vial of Cefazolin Sodium USP equivalent to Cefazolin 500 mg, one ampoule of 10 ml Water for Injection, one sterile disposable syringe (10ml) and one butterfly needle.
Cefazol 1 gm Injection: Each box contains one vial of Cefazolin Sodium USP equivalent to Cefazolin 1 gm, one ampoule of 10 ml Water for Injection, one sterile disposable syringe (10ml) and one butterfly needle.