Tridosil(Azithromycin)
Therapeutic Group: Anti Bacterial
Presentation
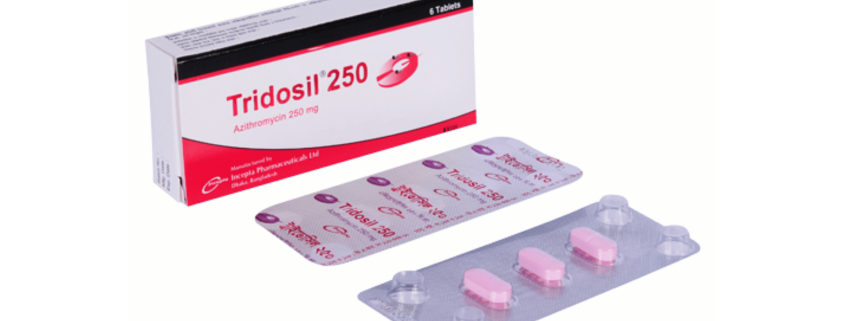
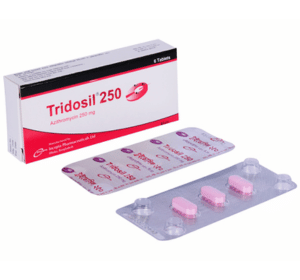
Tridosil 250 Tablet: Each tablet contains Azithromycin Dihydrate USP 262.015 mg equivalent to Azithromycin 250 mg.
Tridosil 500 Tablet: Each tablet contains Azithromycin Dihydrate USP 524.03 mg equivalent to Azithromycin 500 mg.
Tridosil 15 ml Powder for Suspension: After reconstitution, each 5 ml suspension contains Azithromycin Dihydrate USP 209.65 mg equivalent to Azithromycin 200 mg.
Tridosil 30 ml Powder for Suspension: After reconstitution, each 5 ml suspension contains Azithromycin Dihydrate USP 209.65 mg equivalent to Azithromycin 200 mg.
Tridosil 35 ml Powder for Suspension: After reconstitution, each 5 ml suspension contains Azithromycin Dihydrate USP 209.65 mg equivalent to Azithromycin 200 mg.
Tridosil 50 ml Powder for Suspension: After reconstitution, each 5 ml suspension contains Azithromycin Dihydrate USP 209.65 mg equivalent to Azithromycin 200 mg.
Tridosil IV Infusion: Each vial contains lyophilized powder of Azithromycin Dihydrate USP equivalent to Azithromycin 500 mg and each normal saline bottle contains 250 ml 0.9% Sodium Chloride solution. After reconstitution with 250 ml 0.9% Sodium Chloride solution according to direction, each ml contains Azithromycin Dihydrate USP equivalent to Azithromycin 2 mg.
Description
Azithromycin is an azalide antibiotic, a subclass of macrolide antibiotic. It acts by binding to the 50s ribosomal subunit of susceptible microorganisms and thus interfering with microbial protein synthesis. Azithromycin has been shown to be active against most strains in the following microorganisms, both In vitro and in clinical infections:
Gram-positive microorganisms: Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes.
Gram-negative microorganisms: Haemophilus ducreyi, Haemophilus influenzae, Moraxella catarrhalis, Neisseria gonorrhoeae, Escherichia coli.
Other microorganisms: Chlamydia pneumoniae, Chlamydia trachomatis, Mycoplasma pneumoniae, Bacteroides fragilis, Legionella pneumophila, oxoplasma gondii.
Indications
Azithromycin is indicated for infections caused by susceptible organisms in-
Upper respiratory tract infections including sinusitis, pharyngitis and tonsillitis
Lower respiratory tract infections including bronchitis, acute bacterial exacerbations of chronic obstructive pulmonary
disease (COPD)
Otitis media
Skin and soft tissue infections including cellulitis, pyoderma, erysipelas, wound infections
Diarrhea, Shigellosis
Sexually transmitted diseases, especially in the treatment of non-gonococcal urethritis and cervicitis due to Chlamydia trachomatis
Genital ulcer disease in men due to Haemophilus ducreyi (chancroid)
Mild or moderate typhoid due to multiple-antibacterial resistant organisms
Prophylaxis against a-hemolytic (viridans group) streptococcal bacterial endocarditis
Other infections including odontogenic infections, bartonella infections, toxoplasmosis, babesiosis
Dosage & Administration
Azithromycin tablet can be taken with or without food. Azithromycin suspension should be taken at least 1 hour before or 2 hours after meal.
Oral:
Adult:
For respiratory tract infections, otitis media and skin & soft tissue infections: 500 mg once daily for 3 days or an alternative to this as 500 mg once on day 1, followed by 250 mg once daily for next 4 days. For sexually transmitted diseases like genital ulcer, non-gonococcal urethritis and cervicitis due to Chlamydia trachomatis : a single 1 gm (1000 mg) dose. For the treatment of urethritis and cervicitis due to Neisseria gonorrhoeae : a single 2 gm (2000 mg) dose. In typhoid, 500 mg once daily for 7 days. In Cholera, a single 1 gm (1000 mg) dose. In Shigellosis, 500 mg once on day 1, followed by 250 mg once daily for next 4 days.
Children:
Side Effects
Azithromycin is well tolerated with a low incidence of side efects. The side effects include nausea, vomiting, abdominal discomfort (pain/cramps), flatulence, diarrhea, headache, dizziness, and skin rashes and are reversible upon discontinuation of therapy. Reversible elevations in liver transaminases have been observed occasionally. Transient mild reductions in neutrophil counts have occasionally been observed in clinical trials, although causal relationship to Azithromycin has not been established.
Precautions
As with any antibiotic, observation for signs of super infection with non-susceptable organisms, including fungi, is recommended. Precaution should be taken in patients with more severe renal impairment.
Use in Pregnancy & Lactation
Pregnancy: US FDA pregnancy category B. In the animal studies, no evidence of harm to the fetus due to Azithromycin was found. Because animal reproduction studies are not always predictive of human response, Azithromycin should be used during pregnancy only if clearly needed.
Lactation: It is not known whether Azithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Azithromycin is administered to nursing mother.
Drug Interaction
Peak serum levels but not the total extent of absorption were reduced by the presence of magnesium and aluminum-containing antacids. Azithromycin should be taken at least 1 hr before or 2 hrs after these antacids. In patients receiving ergot alkaloids, Azithromycin should be avoided concurrently because of the possibilty of ergotism result in from interaction of Azithromycin with the cytochrome P-450 system However, no cases of such interaction have been reported. Macrolides have been known to increase the plasma concentration of digoxin and cyclosporine. Therefore, if co-administration is necessary caution should be exercised and serum levels of digoxin and cyclosporine should be checked. There have been no pharmacokinetic drug interactions between Azithromycin and warfarin, theophylline, carbamazepine, methylprednisolone and cimetidine.
Over Dose
There are no data available on overdose with Azithromycin. Typical symptoms of overdosage with macrolide antibiotics include hearing loss, severe nausea, vomiting and diarrhoea. Gastric lavage and general supportive measures are indicated.
Commercial Pack
Tridosil 250 Tablet: Each box contains 2 blister strips of 3 tablets.
Tridosil 500 Tablet: Each box contains 4 blister strips of 3 tablets.
Tridosil 15 ml Powder for Suspension: Bottle containing dry powder for 15 ml suspension.
Tridosil 30 ml Powder for Suspension: Bottle containing dry powder for 30 ml suspension.
Tridosil 35 ml Powder for Suspension: Bottle containing dry powder for 35 ml suspension.
Tridosil 50 ml Powder for Suspension: Bottle containing dry powder for 50 ml suspension.
Tridosil IV Infusion: Each pack contains 1 vial of lyophilized powder of Azithromycin 500 mg, 1 bottle of 250 ml normal saline (0.9% Sodium Chloride solution), a 5 ml disposable syringe and 1 infusion set.
Others
Shake the bottle to loosen powder.
15 ml: Add 10 ml ( 2 measuring spoonful) of boiled and cooled water for 15 ml,
30 ml: Add 20 ml (4 measuring spoonful) of boiled and cooled water for 30 ml.
35 ml: Add 23 ml (with the help of supplied cup) of boiled and cooled water for 35 ml, to the dry powder of the bottle.
50 ml: Add 33 ml (with the help of supplied cup) of boiled and cooled water for 50 ml, to the dry powder of the bottle.
For ease of preparation, add water to the bottle in two proportions. Shake well after each addition until all the powder is in suspension.
Note: Shake the suspension well before each use. Keep the bottle tightly closed. The reconstituted suspension should be stored in a cool and dry place, preferably in refrigerator.
Preparation of the solution for intravenous administration Dilution with supplied 250 ml 0.9% normal saline:
1. Draw 5 ml normal saline from the bottle with the syringe.
2. Push this 5 ml normal saline into the vial containing dry powder of Azithromycin and prepare the solution.
3. Draw this prepared solution into the syringe from the vial.
4. Push this solution into the 250 ml bottle of normal saline. Each ml of reconstituted solution contains 2 mg Azithromycin.
Tridosil Powder for IV Infusion can also be diluted with 250 or 500 ml-
1/2 Normal Saline (0.45% sodium chloride), 5% Dextrose in Water, Lactated Ringer\\\’s Solution, 5% Dextrose in 1/2 Normal Saline, (0.45% sodium chloride) with 20 mEq KCl, 5% Dextrose in Lactated Ringer\\\’s Solution, 5% Dextrose in 1/3 Normal Saline (0.3% sodium chloride), or 5% Dextrose in 1/2 Normal Saline (0.45% sodium chloride).